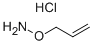
O-ALLYLHYDROXYLAMINE HYDROCHLORIDE
- CAS No.
- 38945-21-0
- Chemical Name:
- O-ALLYLHYDROXYLAMINE HYDROCHLORIDE
- Synonyms
- (Allyloxy)amine hydrochloride;O-ALLYHYDROXYAMINE HYDROCHLORIDE);O-ALLYLHYDROXYLAMINE HYDROCHLORIDE;3-(Aminooxy)prop-1-ene hydrochloride;O-Allylhydroxylamine Hydrochloride >O-prop-2-enylhydroxylamine hydrochloride;O-(2-PROPENYL)HYDROXYLAMINE HYDROCHLORIDE;O-Allylhydroxylamine Hydrochloride;HydroxylaMine, O-2-propenyl-, hydrochloride;O-Allylhydroxylamine hydrochloride >=98.0% (AT)
- CBNumber:
- CB7738618
- Molecular Formula:
- C3H8ClNO
- Molecular Weight:
- 109.55
- MOL File:
- 38945-21-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/6/8 17:06:38
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 3-9 | |||||||||
| HS Code | 2928009090 | |||||||||
| NFPA 704 |
|
O-ALLYLHYDROXYLAMINE HYDROCHLORIDE price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 05983 | O-Allylhydroxylamine hydrochloride ≥98.0% (AT) | 38945-21-0 | 5G | ₹13585.38 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 05983 | O-Allylhydroxylamine hydrochloride ≥98.0% (AT) | 38945-21-0 | 25G | ₹48615.08 | 2022-06-14 | Buy |
| TCI Chemicals (India) | A1449 | O-Allylhydroxylamine Hydrochloride | 38945-21-0 | 1G | ₹3500 | 2022-05-26 | Buy |
| TCI Chemicals (India) | A1449 | O-Allylhydroxylamine Hydrochloride | 38945-21-0 | 5G | ₹10000 | 2022-05-26 | Buy |
O-ALLYLHYDROXYLAMINE HYDROCHLORIDE Chemical Properties,Uses,Production
Preparation
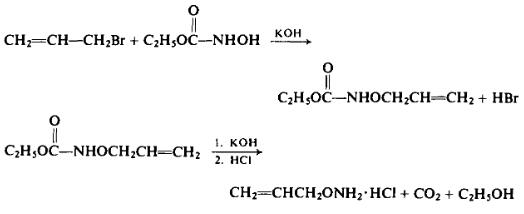
To a solution of 86.8 gm (1.55 moles) of potassium hydroxide in 330 ml of absolute ethanol is added a solution of 159 gm (1.52 moles) of ethyl N-hydroxycarbamate in 330 ml of absolute ethanol. With external cooling to maintain an internal temperature of 25°C to this mixture is added 195.5 gm (1.62 moles) of allyl bromide. After the addition has been completed, the mixture is heated under reflux for 2 hr. After separating the potassium bromide formed during the reaction and washing it with absolute alcohol, the alcoholic solution is evaporated under reduced pressure. The residue is dissolved in ether and then the ether solution is extracted repeatedly with 10% aqueous sodium hydroxide solution. [From the ether solution, on evaporation 25.5 gm (18%) of ethyl N,O-diallylhydroxycar-bamate, b.p. 91-92°C (8.55 mm Hg) may be isolated.] The aqueous extract is acidified with 10% aqueous sulfuric acid and the ethyl O-allylhy-droxycarbamate is extracted with ether. Upon evaporating the ether off, 134.2 gm (61%) of the intermediate product, b.p. 107°C (12.5 mm Hg), is isolated.
In a steam distillation apparatus, 134 gm of ethyl O-allylhydroxy-carbamate is treated with a solution of 120 gm of potassium hydroxide in 280 ml of water. The product is steam-distilled into a receiver containing dilute hydrochloric acid.
The steam distillate is evaporated under reduced pressure. The residue is taken up twice in absolute ethanol and dried by evaporation under reduced pressure. The yield is 91 gm (55%, overall), m.p. 169-170°C. Upon recrystallization from absolute alcohol and dry ether, the melting point is raised to 170.6-170.8°C (172-174°C). Free O-allylhydrox-ylamine has the following reported properties: b.p. 98-99°C, n25D 1.4300.
The problems connected with the preparation of O-arylhydroxylamines by this method have been attributed to the instability of the aryl- substituted aminooxy group to the hydrolytic system used in its preparation. This problem has recently been circumvented by substituting for ethyl N-hydroxycarbamate, t-butyl N-hydroxycarbamate.
O-ALLYLHYDROXYLAMINE HYDROCHLORIDE Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Gagri Global IT Services Pvt. Ltd. | +91-40-27170174, | Telangana, India | 1321 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Reax Chemicals | 08048372701Ext 416 | Hyderabad, India | 4223 | 58 | Inquiry |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 | China | 9337 | 55 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29897 | 58 | Inquiry |
| Jinan Finer Chemical Co., Ltd | +86-531-88989536 +86-15508631887 | China | 2967 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 | China | 12075 | 58 | Inquiry |
38945-21-0(O-ALLYLHYDROXYLAMINE HYDROCHLORIDE)Related Search:
1of4
chevron_right




