Lumefantrine
- CAS No.
- 82186-77-4
- Chemical Name:
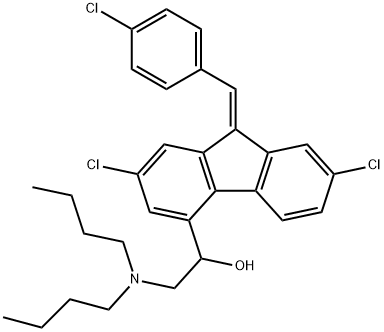
- Lumefantrine
- Synonyms
- LuMefentrine;benflumetol;Benwuchun;CPG-56695;Benflumelol;umefantrine;A + alcohol;Lumefantrine;Benflumentol;Lumefruntrine
- CBNumber:
- CB7854882
- Molecular Formula:
- C30H32Cl3NO
- Molecular Weight:
- 528.94
- MOL File:
- 82186-77-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/18 8:51:57
| Melting point | 129-131°C |
|---|---|
| Boiling point | 642.5±55.0 °C(Predicted) |
| Density | 1.252 |
| storage temp. | 15-25°C |
| solubility | DMSO: ≥20mg/mL |
| pka | 13.44±0.20(Predicted) |
| form | powder |
| color | yellow |
| Merck | 14,5597 |
| CAS DataBase Reference | 82186-77-4(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| WGK Germany | 3 |
| HS Code | 29221950 |
Lumefantrine price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | L5420 | Lumefantrine | 82186-77-4 | 5MG | ₹12329.68 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR2186 | Lumefantrine Pharmaceutical Secondary Standard; Certified Reference Material | 82186-77-4 | 500MG | ₹61410.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | L5420 | Lumefantrine | 82186-77-4 | 25MG | ₹50260.48 | 2022-06-14 | Buy |
| TCI Chemicals (India) | L0256 | Lumefantrine | 82186-77-4 | 5G | ₹8600 | 2022-05-26 | Buy |
| TCI Chemicals (India) | L0256 | Lumefantrine | 82186-77-4 | 25G | ₹9800 | 2022-05-26 | Buy |
Lumefantrine Chemical Properties,Uses,Production
Description
Lumefantrine is a derivative of halofantrine that has been reported to exhibit antimalarial activity when combined with artemether in the treatment of multidrug-resistant Plasmodium falciparium . No evidence of cardiotoxicity has been reported with this combination, which may offer promise for successful treatment of resistant organisms.
Chemical Properties
Yellow Solid
Uses
Lumefantrine has been used:
to study its effect on ex-vivo?Plasmodium falciparum?sensitivity using the tritiated hypoxanthine-based assay
as a standard in the quantification of combined tablet formulation using HPTLC
as a drug molecule in in vitro growth inhibition assay for in vitro B. caballi?growth inhibition studies
Definition
ChEBI: Lumefantrine is an antimalarial drug used in combination with artemether for the treatment of multi-drug resistant strains of falciparum malaria.
Antimicrobial activity
Lumefantrine has marked blood schizonticidal activity against a wide range of plasmodia, including chloroquineresistant P. falciparum. The 50% and 90% effective concentrations (EC50 and EC90) in vitro are similar: <10 and 40 nmol/L, respectively. The racemate and the two enantiomers exhibit similar activities. Blood schizonticidal activity of desbutylbenflumetol is four to five times greater than benflumetol in vitro.
Acquired resistance
Treatment with artemether–lumefantrine can select for polymorphisms in the P. falciparum pfmdr1 gene. Resistance has been selected experimentally in murine malaria.
General Description
Lumefantrine was developed in China. Itsmechanism of action is poorly understood. There is some evidencethat it inhibits the formation of β-hematin by forming acomplex with hemin. Lumefantrine is very lipophilic and is marketed in combination with the lipophilic artemesininderivedartemether.
Pharmaceutical Applications
A dichlorobenzylidene derivative given orally in combination with artemether.
Pharmacokinetics
Bioavailability after oral administration is variable; absorption is substantially increased by co-administration with food, particularly with a high fat content. Peak plasma concentrations occur after 6–8 h. The elimination half-life is 4–6 days. It is almost completely protein bound and metabolized mainly in the liver by CYP3A4.
Clinical Use
Treatment of P. falciparum infections (including mixed infections) in a fixed-dose combination treatment with artemether.
Side effects
The most common adverse effects in combination with artemether include headache, dizziness and gastrointestinal disturbances.
Metabolism
Primaquine is almost totally metabolized by CYP3A4 (99%), with the primary metabolite being carboxyprimaquine. Trace amounts of N-acetylprimaquine plus aromatic hydroxylation and conjugation metabolites also have been reported.
Lumefantrine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| Micro Orgo Chem | +91-91-022-24969510 +91-9082984052 | Mumbai, India | 66 | 58 | Inquiry |
| Chynops Pharma | +919998000404 | Gujarat, India | 39 | 58 | Inquiry |
| Dodhia Group | +91-9619867345 +91-9619867345 | Mumbai, India | 129 | 58 | Inquiry |
| Cipla Ltd | +912224826000 | Maharashtra, India | 133 | 58 | Inquiry |
| Calyx Chemicals and Pharmaceuticals Ltd | +919819317220 | Mumbai, India | 15 | 58 | Inquiry |
| Ipca Laboratories Ltd | +91-2262105000 +91-2262105000 | Maharashtra, India | 61 | 58 | Inquiry |
| Bajaj Healthcare Ltd | +91-2266177400 +91-2266177400 | Maharashtra, India | 86 | 58 | Inquiry |
| Souvin Pharmaceuticals Pvt Ltd | +91-8983015895 +91-8983015895 | Maharashtra, India | 9 | 58 | Inquiry |
| Mangalam Drugs and Organics Ltd | +91-2222616300 +91-2222616200 | Maharashtra, India | 37 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Jigs Chemical ltd | 58 |
| Micro Orgo Chem | 58 |
| Chynops Pharma | 58 |
| Dodhia Group | 58 |
| Cipla Ltd | 58 |
| Calyx Chemicals and Pharmaceuticals Ltd | 58 |
| Ipca Laboratories Ltd | 58 |
| Bajaj Healthcare Ltd | 58 |
| Souvin Pharmaceuticals Pvt Ltd | 58 |
| Mangalam Drugs and Organics Ltd | 58 |
82186-77-4(Lumefantrine)Related Search:
1of4
chevron_right




