BARIUM BROMATE
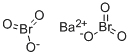
- CAS No.
- 13967-90-3
- Chemical Name:
- BARIUM BROMATE
- Synonyms
- BARIUM BROMATE;barium(2+),dibromate;Bromicacid,bariumsalt;Bisbromic acid barium salt;BARIUM BROMATE CRYSTALLINE;BARIUM BROMATE ISO 9001:2015 REACH
- CBNumber:
- CB8225560
- Molecular Formula:
- BaBr2O6
- Molecular Weight:
- 393.13
- MOL File:
- 13967-90-3.mol
- Modify Date:
- 2024/3/14 15:18:27
| Density | 3,99 g/cm3 |
|---|---|
| solubility | soluble in acetone |
| color | White or colorless crystals or crystalline powder |
| Water Solubility | g/100g solution H2O: 0.286 (0°C), 0.788 (25°C); 5.39 (99.65°C); equilibrium solid phase Ba(BrO3)2 [KRU93] |
| Merck | 13,970 |
| Exposure limits |
ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
| CAS DataBase Reference | 13967-90-3(CAS DataBase Reference) |
| EPA Substance Registry System | Bromic acid, barium salt (13967-90-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS03,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hazard statements | H272-H302-H332 | |||||||||
| Precautionary statements | P221-P262 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 20/22 | |||||||||
| Safety Statements | 28 | |||||||||
| RIDADR | UN 2719 5.1/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | EF8715000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | II | |||||||||
| NFPA 704 |
|
BARIUM BROMATE price More Price(2)
BARIUM BROMATE Chemical Properties,Uses,Production
Description
Barium bromate is used extensively to form other
bromates, including the alkali metal bromates which
are used extensively in Industry. HBrO3, a colorless
liquid, is made by the addition of dilute sulfuric acid
to barium bromate. It dissociates extensively in aqueous
solution and is a strong acid.
The scientific literature contains a few studies of
barium bromate chiefly due to interest in Industry for
its use to prepare other bromate salts.
This salt is available commercially, worldwide.
Chemical Properties
Barium bromate is a white crystalline powder.
Uses
Barium bromate [Ba(BrO3)2] is used as a corrosion inhibitor to prevent rust and as an oxidizing agent and chemical reagent.
Preparation
Barium Bromate may be prepared by reaction of the hydroxide with
sodium bromate:
Ba(OH)2+ 2NaBrO3→Ba(BrO3)2+ 2NaOH
Barium bromate is relatively insoluble, and has
a value of 0.782 g/100 ml of water at 298 K,if recrystallized, it forms a monohydrate,
Ba(BrO3)2·H2O, which occurs as white or colorless, prismatic
crystals.
A new and general method of inducing a precipitation from homogeneous solution has been applied to the separation of barium and strontium. The precipitation of barium bromate, a water-soluble compound, is brought about by a gradual change in the composition of the solvent media. An organic compound, such as methanol or tetrahydrofuran, is allowed to diffuse into the original water solution, thus slowly converting the solution into a water organic mixture. Strontium bromate is about 40 times more soluble in water than barium bromate. The increased solubility plus the greater stability of the strontium MEDTA chelate tend to hold the strontium in solution. The chief advantage of this method over the separation of Sr from Ba by precipitation of barium chromate is in the preparation of barium compounds. Usually, barium must be separated from the chromate before proceeding. However, barium bromate can be easily converted to the oxide, eliminating this separation step.
General Description
A white crystalline solid or powder. Slightly soluble in water and denser than water. Very toxic by ingestion or inhalation. Fire hazard when in contact with organic materials and may explode when heated above 300°F. Used as a corrosion inhibitor and to manufacture other chemicals.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
BARIUM BROMATE is an oxidizing agent. May react violently with combustibles and reducing agents such as textiles, oil, fat, sugar, sawdust, ammonium salts, sulfur, carbon, phosphorus, metal powders and sulfides. A combination of finely divided aluminum with finely divided BARIUM BROMATE can explode by heat, percussion, or friction [Mellor 2:310 1946-47].
Hazard
A poison. Moderate fire risk in contact with organic materials.
Health Hazard
Toxic by ingestion. Inhalation of dust is toxic. Fire may produce irritating, corrosive and/or toxic gases. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. May explode from heat or contamination. Some may burn rapidly. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Safety Profile
Very toxic. Fire hazard by chemical reaction with easily oxidued materials. Explodes at 300℃. Mmures with sulfur are unstable storage hazards; igniting immediately at 91 ℃ and after a 2-1 1 day delay period at room temperature. Incompatible with Al, As, C, Cu, metal sulfides, organic matter, P, and reducing materials. When heated to decomposition it emits toxic fumes of Br-. See also BARIUM COMPOUNDS (soluble) and BROMINE.
Potential Exposure
This material is used as an analytical reagent, oxidizer and corrosion inhibitor.
Shipping
UN2719 Barium bromate, Hazard Class: 5.1; Labels: 5.1—Oxidizer, 6.1—Poisonous materials.
Incompatibilities
A strong oxidizer; keep away from reducing agents. Keep away from oxidizable materials; aluminum, arsenic, carbon, copper, metal sulfides; phosphorus, sulfur, organic, and combustible materials (such as wood, paper, oil, fuels), since violent reactions occur.
BARIUM BROMATE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Krishna Chem Industry | 08048250470Ext 440 | Vadodara, India | 73 | 58 | Inquiry |
| Archit Meta Chem | 08048371779Ext 300 | Ahmedabad, India | 64 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 8744 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 57511 | 58 | Inquiry |
| ABCR GmbH & CO. KG | 49 721 95061 0 | Germany | 6846 | 75 | Inquiry |
| Celtic Chemicals Ltd. | +44 (1656) 749-358 | United Kingdom | 225 | 50 | Inquiry |
| Shandong Xiya Chemical Co., Ltd. | 4009903999 13395398332 | China | 20807 | 60 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | 029-61856358 15229202216 | China | 10011 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Alfa Aesar | 58 |
| Krishna Chem Industry | 58 |
| Archit Meta Chem | 58 |
| Chongqing Chemdad Co., Ltd | 58 |
| Shaanxi Dideu Medichem Co. Ltd | 58 |
| Aladdin Scientific | 58 |
| ABCR GmbH & CO. KG | 75 |
| Celtic Chemicals Ltd. | 50 |
| Shandong Xiya Chemical Co., Ltd. | 60 |
| Shaanxi Dideu Medichem Co. Ltd | 58 |
13967-90-3(BARIUM BROMATE)Related Search:
1of4
chevron_right




