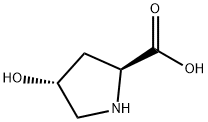
L-Hydroxyproline
- CAS No.
- 51-35-4
- Chemical Name:
- L-Hydroxyproline
- Synonyms
- TRANS-4-HYDROXY-L-PROLINE;HYDROXYPROLINE;(2S,4R)-4-HYDROXYPYRROLIDINE-2-CARBOXYLIC ACID;Hyp;4-Hydroxyproline;4-HYDROXY-L-PROLINE;H-HYP-OH;4-HYDROXYPYRROLIDINE-2-CARBOXYLIC ACID;HYDROXY-L-PROLINE;4R-4-hydroxyproline
- CBNumber:
- CB8357870
- Molecular Formula:
- C5H9NO3
- Molecular Weight:
- 131.13
- MOL File:
- 51-35-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 273 °C (dec.)(lit.) |
|---|---|
| alpha | -75.5 º (c=5, H2O) |
| Boiling point | 242.42°C (rough estimate) |
| Density | 1.3121 (rough estimate) |
| bulk density | 610kg/m3 |
| vapor density | 4.5 (vs air) |
| refractive index | -75.5 ° (C=4, H2O) |
| storage temp. | Store below +30°C. |
| solubility | H2O: 50 mg/mL |
| form | Crystals or Crystalline Powder |
| pka | 1.82, 9.66(at 25℃) |
| color | White |
| Odor | Odorless |
| PH | 5.5-6.5 (50g/l, H2O, 20℃) |
| optical activity | [α]25/D 75.6°, c = 1 in H2O |
| Water Solubility | 357.8 g/L (20 º C) |
| Merck | 14,4840 |
| BRN | 471933 |
| InChIKey | PMMYEEVYMWASQN-DMTCNVIQSA-N |
| LogP | -0.350 (est) |
| CAS DataBase Reference | 51-35-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Hydroxyproline(51-35-4) |
| EPA Substance Registry System | trans-4-Hydroxy-L-proline (51-35-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P271-P280 | |||||||||
| Hazard Codes | Xi,Xn | |||||||||
| Risk Statements | 36/37/38-22 | |||||||||
| Safety Statements | 24/25-36/37/39-27-26 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TW3586500 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 29339990 | |||||||||
| NFPA 704 |
|
L-Hydroxyproline price More Price(23)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | H5534 | trans-4-Hydroxy-L-proline BioReagent, suitable for cell culture, ≥98.5% | 51-35-4 | 10MG | ₹2955.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | H5534 | trans-4-Hydroxy-L-proline BioReagent, suitable for cell culture, ≥98.5% | 51-35-4 | 25G | ₹23522.73 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | H5534 | trans-4-Hydroxy-L-proline BioReagent, suitable for cell culture, ≥98.5% | 51-35-4 | 100G | ₹63066.45 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1939 | Hydroxyproline Pharmaceutical Secondary Standard; Certified Reference Material | 51-35-4 | 500MG | ₹15826.15 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | H5534 | trans-4-Hydroxy-L-proline BioReagent, suitable for cell culture, ≥98.5% | 51-35-4 | 1KG | ₹319705.55 | 2022-06-14 | Buy |
L-Hydroxyproline Chemical Properties,Uses,Production
Description
A non-essential amino acid. Can be isolated from gelatin. Hydroxyproline has not been reported as added to food in any of the NAS surveys.
Chemical Properties
White crystalline powder
Uses
A natural constituent of animal structural proteins such as collagen and elastin. Several microorganisms producing proline trans-4- and cis-3-hydroxylase were discovered and these enzymes were applied to the industrial production of trans-4- and cis-3-hydroxy-L-proline.
Production Methods
In the past L-Hydroxyproline was isolated by hydrolysis of animal collagen, e. g. gelatine or collagen, of which it is a major constituent. L-Hydroxyproline is an unnatural amino acid which is made in the body by hydroxylation of L-Proline. The presence of L-Hydroxyproline and proline are key to maintaining the stability of the tight collagen helix.
Today L-Hydroxyproline is manufactured by bio-catalysed hydroxylation of proline in bacteria. Together with its other isomers, L-Hydroxyproline is also used as an inter mediate for a range of pharmaceutical active ingredients.
Produced by hydroxylation of L-proline after protein synthesis. It is contained rich in collagen and elastin. Known to stabilization of triple-helix structure of collagen. Widely used as an intermediate for medicines.
Definition
ChEBI: An optically active form of 4-hydroxyproline having L-trans-configuration.
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Purification Methods
Crystallise it from MeOH/EtOH (1:1). Separation from normal allo-isomer can be achieved by crystallisation of the copper salts [see Levine Biochemical Preparations 8 114 1961]. Separation from proline is achieved via the crystalline picrate, CdCl2, or acid ammonium rhodanate salts [see Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 2182 1961, Kapfhammer & Mohn Z Physiol Chem 306 76 1956]. [Beilstein 22/5 V 7.]
L-Hydroxyproline Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| BTC pharm India | +918790379245 | AndhraPradesh, India | 1262 | 58 | Inquiry |
| ARRAKIS INDUSTRIES LLP | +91 74995 32711 | Maharashtra, India | 1353 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| BTC pharm India | +918790379245 | Telangana, India | 412 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6768 | 58 | Inquiry |
| Scientific OEM | +91-22- 2343 7546 / 2341 3094 | New Delhi, India | 1994 | 38 | Inquiry |
| ALPHA CHEMIKA | +91-22-22061123 +91-22-66382501 | Mumbai, India | 1679 | 43 | Inquiry |
| Parth Antibiotics Pvt Ltd. | +91 (22) 2562-8329 | New Delhi, India | 173 | 50 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6700 | 30 | Inquiry |
| Lalchand Bhimraj Group | 91-44-25354424 | Tamil Nadu, India | 50 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| BTC pharm India | 58 |
| ARRAKIS INDUSTRIES LLP | 58 |
| JSK Chemicals | 58 |
| BTC pharm India | 58 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |
| Scientific OEM | 38 |
| ALPHA CHEMIKA | 43 |
| Parth Antibiotics Pvt Ltd. | 50 |
| CHEMSWORTH | 30 |
| Lalchand Bhimraj Group | 58 |
Related articles
- L-Hydroxyproline: Applications, Supplements and Benefits
- L-Hydroxyproline (Hydroxyproline, Hyp) is an important amino acid, accounting for about 13% of the collagen content of amino a....
- Dec 16,2024
- Synthesize and physical properties of L-hydroxyproline
- L-Hydroxyproline has a special flavor and can be used as a fragrance raw material.
- Jul 29,2022





