Ironpentacarbonyl
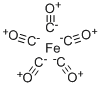
- CAS No.
- 13463-40-6
- Chemical Name:
- Ironpentacarbonyl
- Synonyms
- Fe(CO)5;PENTACARBONYLIRON;IRON(0)CARBONYL;ferpentacarbonyle;pentacarbonyl-iro;Pentacarbonyleisen;ironcarbonylfe(co)5;IRON(0)PENTACARBONYL;PENTACARBONYLIRON(0);carbon monoxide:iron
- CBNumber:
- CB8475886
- Molecular Formula:
- 5CO.Fe
- Molecular Weight:
- 195.9
- MOL File:
- 13463-40-6.mol
- Modify Date:
- 2023/10/17 17:11:55
| Melting point | -20 °C |
|---|---|
| Boiling point | 103 °C(lit.) |
| Density | 1.49 g/mL at 25 °C(lit.) |
| vapor density | 6.74 (vs air) |
| vapor pressure | 35 mm Hg ( 25 °C) |
| refractive index |
n |
| Flash point | 5 °F |
| storage temp. | 2-8°C |
| solubility | insoluble in H2O; soluble in ethyl ether, benzene, acetone |
| form | Liquid Which May Contain Particulate Matter |
| Specific Gravity | 1.490 |
| color | Yellow-orange to brown |
| Water Solubility | Soluble in nickel tetracarbonyl and organic solvents. Insoluble in water |
| Sensitive | Air Sensitive |
| Hydrolytic Sensitivity | 3: reacts with aqueous base |
| Merck | 14,5099 |
| Exposure limits |
TLV-TWA: 0.1 ppm (~0.8 mg(Fe)/m3) (ACGIH), 0.01 ppm (MSHA) TLV-STEL: 0.2 ppm (~1.6 mg(Fe)/m3) (ACGIH). |
| Stability | Moisture Sensitive |
| LogP | 3 at 25℃ |
| CAS DataBase Reference | 13463-40-6(CAS DataBase Reference) |
| EPA Substance Registry System | Iron pentacarbonyl (13463-40-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hazard statements | H225-H300-H310-H330 | |||||||||
| Precautionary statements | P210-P280h-P302+P352-P304+P340-P310 | |||||||||
| Hazard Codes | F,T+ | |||||||||
| Risk Statements | 11-24-26/28 | |||||||||
| Safety Statements | 16-26-28-36/37/39-45 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.1 ppm (0.23 mg/m3), STEL: 0.2 ppm (0.45 mg/m3) | |||||||||
| RIDADR | UN 1994 6.1/PG 1 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | NO4900000 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 29310099 | |||||||||
| Toxicity | LD50 in mice, rats (mg/l): 2.19, 0.91 inhalation for 30 min. (Sunderman) | |||||||||
| IDLA | 0.4 ppm | |||||||||
| NFPA 704 |
|
Ironpentacarbonyl price More Price(2)
Ironpentacarbonyl Chemical Properties,Uses,Production
Chemical Properties
yellow-orange to brown liquid which
Uses
To make finely divided iron, so-called carbonyl iron, which is used in the manufacture of powdered iron cores for high frequency coils used in the radio and television industry; as antiknock agent in motor fuels; as catalyst and reagent in organic reactions.
General Description
A yellow to dark red liquid. Insoluble in water and denser than water. Very toxic by inhalation, ingestion, and skin absorption. Flash point 5°F. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Ironpentacarbonyl is spontaneously flammable in air, [R. Kamo, IIT Progs. Rept. 1, p. 23(1962)]. Insoluble in water.
Reactivity Profile
Organometallics, such as Ironpentacarbonyl, are reactive with many other groups. Incompatible with acids and bases. Organometallics are good reducing agents and therefore incompatible with oxidizing agents. Often reactive with water to generate toxic or flammable gases. A brown pyrophoric powder is produced by the combination of the carbonyl with acetic acid containing greater than 5% of water.
Health Hazard
Toxicity of Ironpentacarbonyl is high via all routes of entry. Cyanosis (bluish discoloration of skin) and circulatory collapse may occur after exposure. Death may result. Pneumonitis and injury to the kidneys, liver, and central nervous system may also occur.
Fire Hazard
Ironpentacarbonyl may be ignited by heat, sparks, or flames. Vapors may travel to ignition source and flash back. Containers may explode in the heat of fire. Evolution of carbon monoxide may create a poison hazard. Ironpentacarbonyl presents a vapor explosion and poison hazard indoors, outdoors, or in sewers. Evolves carbon monoxide on exposure to air or to light. Emits carbon monoxide when heated to decomposition. Avoid acetic acid, water, nitrogen oxide, transition metal halides, and zinc and Ironpentacarbonyl burns in air. Decomposes in acids and alkalies. Protect from light and air.
Purification Methods
It is a pale yellow viscous liquid which is PYROPHORIC and readily absorbed by the skin. HIGHLY TOXIC (protect from light and air). It should be purified in a vacuum line by distilling and collecting in a trap at -96o (toluene-Dry ice slush). It has been distilled at atmospheric pressure (use a very efficient fume cupboard). At 180o/atmospheric pressure it decomposes to give Fe and CO. In UV light in pet ether it forms Fe2(CO)9 (see previous entry). [Hagen et al. Inorg Chem 17 1369 1978, Ewens et al. Trans Faraday Soc 35 6811 1939.]
Ironpentacarbonyl Preparation Products And Raw materials
Raw materials
1of2
chevron_right




