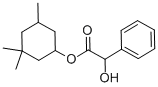
CYCLANDELATE
- CAS No.
- 456-59-7
- Chemical Name:
- CYCLANDELATE
- Synonyms
- bs572;Cydel;Natil;andeL;BS 572;Capilan;Anaspat;Lejopan;Dilatan;Novodil
- CBNumber:
- CB8668024
- Molecular Formula:
- C17H24O3
- Molecular Weight:
- 276.37
- MOL File:
- 456-59-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/12/19 16:21:16
| Melting point | 55.0-56.5° |
|---|---|
| Boiling point | bp14 192-194° |
| Density | 1.0535 (rough estimate) |
| refractive index | 1.5490 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| Water Solubility | Insoluble in water |
| solubility | Chloroform (Slightly), Methanol (Slightly, Sonicated) |
| form | powder to crystal |
| pka | 12.13±0.20(Predicted) |
| color | White to Almost white |
| Merck | 14,2704 |
| EPA Substance Registry System | Benzeneacetic acid, .alpha.-hydroxy-, 3,3,5-trimethylcyclohexyl ester (456-59-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P |
| RTECS | OO8200000 |
| HS Code | 2918191350 |
| Toxicity | LD50 oral in rat: 5gm/kg |
CYCLANDELATE Chemical Properties,Uses,Production
Physical properties
Appearance: white or almost white amorphous powder, special smell, and bitter taste. Solubility: very soluble in ethanol or acetone and almost insoluble in water. Melting point: 50–62 °C.
History
The chemical synthesis of cyclandelate was first synthesized by Funcke et al. from Elan Corporation in Ireland using a-hydroxyphenylacetic acid and cis-3,3,5-cycloalkyl cyclohexanol. The raw material of mandelic acid was obtained by hydrolysis after benzaldehyde reacted to sodium cyanide. However, sodium cyanide is hypertoxic, and because of its unique structure of a-hydroxy acid, it is easy to decompose under the acid condition, which leads to more by-products and low yield. A series of improved methods have been developed, which can reduce the environmental pollution under the premise of ensuring the yield of Zn/HCOONH4/ C2H5OH system . The method was used to synthesize cyclandelate since then. However, this drug has not yet been approved by the Food and Drug Administration in the United States, Canada, and other countries because it easily causes white blood cell deficiency. It has been withdrawn from the market after drug approval in Japan, France, and other countries in the 1970s.
Uses
vasodilator
Indications
The indications of cyclandelate are arteriosclerosis obliterans, acrocyanosis, cerebral arteriosclerosis, cerebral insufficiency, cerebrovascular disease, brain trauma, and post-traumatic brain syndrome.
Definition
ChEBI: The ester obtained by formal condensation of mandelic acid and 3,3,5-tricyclohexanol. It is a direct-acting smooth muscle relaxant used to dilate blood vessels.
World Health Organization (WHO)
Cyclandelate is a papaverine type spasmolytic and vasodilating drug intended for symptomatic treatment of various peripheral vascular disorders, such as intermittent claudication in arteriosclerosis obliterans as well as a treatment for cognitive dysfunction in patients suffering from senile dementia of the multi-infarct or Alzheimer's type. Cyclandelate remains registered in several countries.
Pharmacology
The chemical structure and effect of cyclandelate are similar to papaverine. It can directly relax vascular smooth muscle and relieve the spasm of ileum and uterus smooth muscle induced by acetylcholine, histamine, and barium chloride in guinea pig. This effect is three to five times stronger than papaverine. Cyclandelate can also expand the cardiovascular, cerebrovascular, and renal blood vessels and limb peripheral vascular and coronary artery, increase blood flow, and promote blood circulation . It can also increase the tolerance to hypoxia, but the effect on human cerebral blood flow has not been confirmed. It was reported that cyclandelate can promote collateral circulation but has little effect on respiration, blood pressure, cardiac output, and myocardial oxygen consumption. It is safe for long-term administration .
Clinical Use
Cyclandelate can be used for clinical treatment of cerebral arteriosclerosis, cerebral vascular accident and its sequelae, post-traumatic brain syndrome, coronary arteriosclerosis, hypertensive heart disease, Raynaud’s disease, thromboangiitis obliterans, acrocyanosis, and Meniere’s disease .
CYCLANDELATE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | China | 12459 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29897 | 58 | Inquiry |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | China | 8826 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 | China | 11718 | 58 | Inquiry |
456-59-7(CYCLANDELATE)Related Search:
1of4
chevron_right




