Boron trichloride
- CAS No.
- 10294-34-5
- Chemical Name:
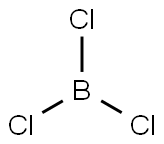
- Boron trichloride
- Synonyms
- BCl3;Trichloroborane;Boron trichL;Trichloroboron;Boron chloride;chloruredebore;trichloro-boran;trichloro-Boron;Chlorure de bore;Boron Trichlorid
- CBNumber:
- CB8852623
- Molecular Formula:
- BCl3
- Molecular Weight:
- 117.17
- MOL File:
- 10294-34-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | −107 °C(lit.) |
|---|---|
| Boiling point | 12.5 °C(lit.) |
| Density | 1.326 g/mL at 25 °C |
| vapor density | 4.05 (vs air) |
| vapor pressure | 29.72 psi ( 55 °C) |
| Flash point | 84 °F |
| storage temp. | 2-8°C |
| solubility | Miscible with dichloromethane, ethanol, carbon tetrachloride, diethyl ether, dimethyl formamide, aromatic solvents, saturated and halogenated hydrocarbon. |
| form | Solution |
| color | White |
| Water Solubility | decomposes |
| Sensitive | Moisture Sensitive |
| Merck | 14,1348 |
| Exposure limits |
ACGIH: TWA 50 ppm OSHA: TWA 25 ppm; STEL 125 ppm NIOSH: IDLH 2300 ppm |
| Stability | Unstable. Incompatible with metals. Reacts violently with water. Reacts vigorously with aniline, phosphine, dinitrogen tetroxide. Fumes in moist air. |
| CAS DataBase Reference | 10294-34-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Borane, trichloro-(10294-34-5) |
| EPA Substance Registry System | Boron trichloride (10294-34-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS05,GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H300-H311+H331-H314-H360FD-H370 | |||||||||
| Precautionary statements | P201-P210-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | T+,T,N,F | |||||||||
| Risk Statements | 14-26/28-36/37/38-40-67-65-62-51/53-48/20-34-11-50/53-26/27/28-63-39/23/24/25-24-21-10 | |||||||||
| Safety Statements | 9-26-28-36/37/39-45-8-61-38-28A-16-1-60-33-23-7/9-62-36/37 | |||||||||
| RIDADR | UN 3390 6.1/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | ED1925000 | |||||||||
| F | 10-21 | |||||||||
| Hazard Note | Very toxic | |||||||||
| TSCA | Yes | |||||||||
| DOT Classification | 2.3, Hazard Zone C (Gas poisonous by inhalation) | |||||||||
| HazardClass | 2.3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28121049 | |||||||||
| Hazardous Substances Data | 10294-34-5(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
Boron trichloride price More Price(30)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 91379 | Boron trichloride solution 12% in methanol, for GC derivatization, LiChropur? | 10294-34-5 | 100ML | ₹12023.2 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 91379 | Boron trichloride solution 12% in methanol, for GC derivatization, LiChropur? | 10294-34-5 | 10X1ML | ₹17981.6 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 91379 | Boron trichloride solution 12% in methanol, for GC derivatization, LiChropur? | 10294-34-5 | 500ML | ₹29140.3 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 678732 | Boron trichloride solution 1.0?M in toluene | 10294-34-5 | 100ML | ₹8538.6 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 348325 | Boron trichloride solution 1.0?M in heptane | 10294-34-5 | 100ML | ₹5905.2 | 2022-06-14 | Buy |
Boron trichloride Chemical Properties,Uses,Production
Description
Boron trichloride is a colorless, acid gas that fumes in the presence of moist air. It is packaged in steel cylinders as a liquid under its own vapor pressure of 19.1 psia (132 kPa, abs) at 70°F (21.1°C). It reacts with water or moist air to produce hydrochloric and boric acid.
Chemical Properties
Boron trichloride is a colorless gas with a pungent odor. It reacts violently with water, and on decomposition and hydrolysis yields hydrochloric and boric acid. It has a pungent, highly irritating odor. Occupational exposure to boron and boron compounds can occur in industries that produce special glass, washing powder, soap and cosmetics, leather, cement, etc.
Uses
Boron trichloride is used in the refining of aluminum,
copper, magnesium, and zinc to remove
oxides, nitrides, and carbides trom the molten
metal. Carbon monoxide, hydrogen, and nitrogen
can be removed from an aluminum melt by
treating with boron trichloride. It also improves
the tensile strength of aluminum and will allow
remelting without a major change in the grain
structure.
The electronic industry benefits trom boron
trichloride in many applications. It is used in the
production of optical fibers, as a p-type dopant
for thermal diffusion in silicon, and for ion implantation.
Application
One of the most important uses of Boron trichloride is in the preparation of boron
fibers ( Fibers, 13. Refractory Fibers). Typically an electrically heated tungsten filament is
passed through a chamber containing BCl3 and
hydrogen. The BCl3 is reduced, and boron is
deposited on the filament, producing a stiff,
strong boron fiber.Boron trichloride, like the trifluoride, has been
used as a Lewis acid catalyst in organic synthesis
in the polymerization of olefins and phosphazines, as well as in catalysis of other organic
reactions. Boron trichloride is also used in plasma etching of aluminum and silicon, in semiconductor manufacturing, and as a source of boron
for chemical vapor deposition. Steel is boronized
by contacting it with a reactive mixture of hydrogen, hydrocarbons, and BCl3 at high
temperatures.
General Description
Boron trichloride appears as a colorless gas with a pungent odor. Fumes irritate the eyes and mucous membranes. Corrosive to metals and tissue and is toxic. Under prolonged exposure to fire or intense heat, the containers may rupture violently and rocket. Used as a catalyst in chemical manufacture, in soldering fluxes, and for many other uses.
Air & Water Reactions
Fumes in air, including moisture in air and soil, to form hydrochloric acid [Merck 11th ed. 1989]. Reacts vigorously with water and forms hydrochloric acid fumes and boric acid.
Reactivity Profile
Boron trichloride vigorously attacks elastomers and packing materials. Contact with Viton, Tygon, Saran and natural and synthetic rubbers is not recommended. Highly corrosive to most metals in the presence of moisture. Reacts energetically with nitrogen dioxide/dinitrogen tetraoxide, aniline, phosphine, triethylsilane, or fat and grease [Mellor 5:132 1946-47]. Reacts exothermically with chemical bases (examples: amines, amides, inorganic hydroxides).
Fire Hazard
When heated to decomposition, Boron trichloride emits toxic fumes of chlorides. Boron trichloride will react with water or steam to produce heat, and toxic and corrosive fumes. In hot water, decomposes to hydrochloric acid and boric acid. Fumes and hydrolyzes in moist air to form hydrochloric acid and oily, irritating corrosives. Avoid aniline, hexafluorisopropylidene amino lithium, nitrogen dioxide, phosphine, grease, organic matter, and oxygen. Nitrogen peroxide, phosphine, fat or grease react energetically with Boron trichloride . Oxygen and Boron trichloride react vigorously on sparking. Boron trichloride and aniline react violently in the absence of a coolant or diluent. Stable.
Potential Exposure
Used in refining of aluminum, magnesium, copper alloys, and in polymerization of styrene. Manufacture and purification of boron; catalyst in organic reactions; semiconductors; bonding of iron or steel; purification of metal alloys to remove oxides, nitrides, and carbides; chemical intermediate for boron filaments; soldering flux; electrical resistors; and extinguishing magnesium fires in heat treating furnaces.
storage
Boron trichloride cylinders should be protected from physical damage. The cylinders should be stored upright and fi rmly secured to prevent falling or being knocked over, in a cool, dry, well-ventilated area of non-combustible construction away from heavily traffi cked areas and emergency exits
Purification Methods
Purify it (from chlorine) by passage through two mercury-filled bubblers, then fractionally distil it under a slight vacuum. In a more extensive purification the nitrobenzene addition compound is formed by passage of the gas over nitrobenzene in a vacuum system at 10o. Volatile impurities are removed from the crystalline yellow solid by pumping at -20o, and the BCl3 is recovered by warming the addition compound at 50o. Passage through a trap at -78o removes entrained nitrobenzene, the BCl3 finally condensing in a trap at -112o [Brown & Holmes J Am Chem Soc 78 2173 1956]. Also purify it by condensing it into a trap cooled in acetone/Dry-ice, where it is pumped for 15minutes to remove volatile impurities. It is then warmed, recondensed and again pumped. [Gamble Inorg Synth III 27 1950.] TOXIC.
Incompatibilities
Incompatible with lead, graphiteimpregnated asbestos, potassium, sodium. Vigorously attacks elastomers, packing materials, natural and synthetic rubber; viton, tygon, saran, silastic elastomers. Avoid aniline, hexafluorisopropylidene amino lithium, nitrogen dioxide, phosphine, grease, organic matter; and oxygen. Nitrogen peroxide, phosphine. Fat or grease react vigorously with boron trichloride. It reacts with water or steam to produce heat, boric acid, and corrosive hydrochloric acid fumes. Oxygen and boron trichloride react vigorously on sparking. Attacks most metals in the presence of moisture.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Nonrefillable cylinders should be disposed of in accordance with local, state, and federal regulations. Allow remaining gas to vent slowly into atmosphere in an unconfined area or exhaust hood. Refillabletype cylinders should be returned to original supplier with any valve caps and outlet plugs secured and valve protection caps in place.
Precautions
Boron trichloride vigorously attacks elastomers and packing materials, natural and synthetic rubbers. It also reacts energetically with nitrogen dioxide/dinitrogen tetraoxide, aniline, phosphine, triethylsilane, or fat and grease. It reacts exothermically with chemical bases such as amines, amides, and inorganic hydroxides. Occupational workers should use gloves of neoprene or butyl rubber, PVC or polyethylene, safety goggles, or glasses and face shield, and safety shoes.
Boron trichloride Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Almas Life Sciences | +919441456585 | Hyderabad, India | 54 | 58 | Inquiry |
| SURVIVAL TECHNOLOGIES LIMITED | +91 8108285261 | Gujarat, India | 294 | 58 | Inquiry |
| ALS INDIA LIFE SCIENCES | +91-8977036379 +91-8977036379 | Hyderabad, India | 1878 | 58 | Inquiry |
| Organo Metallics | +91-9391016448 +91-9705345345 | Telangana, India | 32 | 58 | Inquiry |
| Arnavi Laboratory | +91-7780703633 +91-7780703633 | Telangana, India | 83 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Spectrochem Private Limited | 08048372608Ext 246 | Maharashtra, India | 1055 | 58 | Inquiry |
| Uttam Air Products Private Limited | 91-11-46194400 | Haryana, India | 3 | 58 | Inquiry |
| Survival Technologies Pvt., Ltd. (STPL) | 91-22-61902008 | Maharashtra, India | 119 | 58 | Inquiry |
| Anantco Enterprises Pvt., Ltd. | 91-22-67685000 | Maharashtra, India | 71 | 58 | Inquiry |
Related articles
- What is Boron trichloride used for?
- Boron trichloride (chemical formula: BCl3) is an inorganic compound commonly used in dry etching and chemical vapor deposition....
- Nov 15,2024
10294-34-5(Boron trichloride )Related Search:
1of4
chevron_right




