vanadium dichloride oxide

- CAS No.
- 10213-09-9
- Chemical Name:
- vanadium dichloride oxide
- Synonyms
- Vanadyl dichloride;VANADYLOXYCHLORIDE;dichlorooxovanadium;VANADYLOXYDICHLORIDE;dichlorooxo-Vanadium;Vanadium oxydichloride;Vanadium, dichlorooxo-;Dichlorooxovanadium(IV);Vanadium(IV) oxychloride;vanadium dichloride oxide
- CBNumber:
- CB8930824
- Molecular Formula:
- Cl2OV+
- Molecular Weight:
- 137.8469
- MOL File:
- 10213-09-9.mol
- Modify Date:
- 2024/7/11 15:06:36
| Density | 2.88 |
|---|---|
| solubility | reacts with H2O; soluble in ethanol |
| form | green hygroscopic crystals |
| color | green hygroscopic crystals, crystalline |
| Water Solubility | slowly decomposed in water; soluble absolute alcohol, glacial acetic acid [MER06] |
| EPA Substance Registry System | Vanadium, dichlorooxo- (10213-09-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS05 |
|---|---|
| Signal word | Danger |
| Hazard statements | H302-H314 |
| Precautionary statements | P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501-P264-P270-P301+P312-P330-P501 |
| RIDADR | 2923 |
| HazardClass | 8 |
| PackingGroup | III |
vanadium dichloride oxide Chemical Properties,Uses,Production
Chemical Properties
Green, deliquescent crystal. Usual technical product is a dark-green, syrupymass, 76–82% pure, or a solution. Slowly decomposed by water; soluble in water, alcohol, and acetic acid; may react violently with potassium.
Uses
Vanadium Dichloride Oxide has been used as mordant in printing fabrics.
Preparation
A thoroughly ground mixture of 3.6 g. of dry V2O5 and 9.4 g. of VCl3 is placed at the closed end of a l-m.-longtube, and 0.9 ml. of VOCl3 is then added. The upper part of the tube must be free of traces of these substances. The tube, filled with air, is melt-sealed, and is covered along its entire length with a sheet-metal jacket; its lower third, in a slightly inclined position, is then heated to about 600℃ with a tubular electric furnace. The sheet-metal jacket provides a temperature gradient along which the product VOCl2 sublimes out of the hot reaction zone. This procedure requires at least 4-5 days. However, the yield can be increased by longer heating time. Green needlelike crystals of VOCl2 are deposited in the cold part of the tube. The tube is opened at a suitable spot; the product is suspended in petroleum ether, ethyl ether or CCl4 to dissolve some adhering VOCl3, and then suction-filtered on a coarse fritted-glass filter. The relatively coarse filter separates the VOCl2 crystals from traces of finely divided hydrolysis products. The VOCl2 is freed of adhering solvent and stored under anhydrous conditions.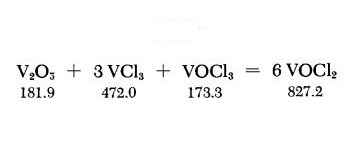
Hazard
Strong irritant to tissue.
vanadium dichloride oxide Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 9003 | 58 | Inquiry |
| HONEST JOY HOLDINGS LIMITED | +86-755-26404303 | United States | 6702 | 54 | Inquiry |
| Hangzhou Ocean Chemical Co., Ltd | 0571-88025872 13588138709 | China | 6104 | 0 | Inquiry |
| Shaanxi Dideu Newmaterial Co., Ltd. | 029-63373950 15353716720 | China | 10011 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Shaanxi Didu New Materials Co. Ltd | 58 |
| HONEST JOY HOLDINGS LIMITED | 54 |
| Hangzhou Ocean Chemical Co., Ltd | 0 |
| Shaanxi Dideu Newmaterial Co., Ltd. | 58 |





