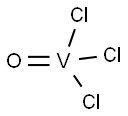
VANADIUM(V) TRICHLORIDE OXIDE
- CAS No.
- 7727-18-6
- Chemical Name:
- VANADIUM(V) TRICHLORIDE OXIDE
- Synonyms
- VOCl3;Vanadium oxychloride;VANADYL CHLORIDE;vanadylic chloride;Vanadyl trichloride;VANADYL(V) CHLORIDE;Vanadyl(IV) chloride;trichlorooxo-vanadiu;trichlorooxovanadium;vanadylchloride(vocl3)
- CBNumber:
- CB8726542
- Molecular Formula:
- Cl3OV
- Molecular Weight:
- 173.3
- MOL File:
- 7727-18-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/11/28 16:31:44
| Melting point | −77 °C(lit.) |
|---|---|
| Boiling point | 126-127 °C(lit.) |
| Density | 1.84 g/mL at 25 °C(lit.) |
| vapor pressure | 13.8 mm Hg ( 20 °C) |
| refractive index | 1.6300 |
| Flash point | 126-127°C |
| solubility | Miscible with benzene, dichloromethane and hexane. |
| form | Liquid |
| color | Yellow |
| Specific Gravity | 1.84 |
| Water Solubility | decomposes in H2O to vanadic acid and HCl; soluble methanol, ether, acetone, acids [KIR83] [MER06] |
| Sensitive | Moisture Sensitive |
| Merck | 14,9927 |
| Dielectric constant | 3.6(26℃) |
| Exposure limits | NIOSH: Ceiling 0.05 mg/m3 |
| LogP | 0 at 20℃ |
| CAS DataBase Reference | 7727-18-6(CAS DataBase Reference) |
| EPA Substance Registry System | Vanadium, trichlorooxo-, (T-4)- (7727-18-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H314 | |||||||||
| Precautionary statements | P270-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338-P363 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 14-25-34 | |||||||||
| Safety Statements | 26-27-28-36/37/39-45 | |||||||||
| RIDADR | UN 2443 8/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | YW2975000 | |||||||||
| F | 10-21 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 orally in rats: 0.14 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
VANADIUM(V) TRICHLORIDE OXIDE price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 200891 | Vanadium(V) oxychloride 99% | 7727-18-6 | 100G | ₹8508.45 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 200891 | Vanadium(V) oxychloride 99% | 7727-18-6 | 100G | ₹8508.45 | 2022-06-14 | Buy |
| ALFA India | ALF-081118-36 | Vanadium(V) trichloride oxide, V+5 28.5% min | 7727-18-6 | 500g | ₹34818 | 2022-05-26 | Buy |
| ALFA India | ALF-081118-22 | Vanadium(V) trichloride oxide, V+5 28.5% min | 7727-18-6 | 100g | ₹6468 | 2022-05-26 | Buy |
| ALFA India | ALF-081118-09 | Vanadium(V) trichloride oxide, V+5 28.5% min | 7727-18-6 | 10g | ₹3001 | 2022-05-26 | Buy |
VANADIUM(V) TRICHLORIDE OXIDE Chemical Properties,Uses,Production
Chemical Properties
Lemon-yellow liquid. Nonionizing solvent. Dissolves most nonmetals; dissolves and/or reacts with many organic compounds; hydrolyzes in moisture.
Uses
Vanadium(V) trichloride oxide is used as a catalyst in olefin polymerization reaction, especially for the production of ethylene-propylene rubber. It is also used to synthesis organovanadium compounds and as a mordant in dyeing. It acts as a precursor to prepare vanadium pentoxide, vanadyl dichloride and dioxovanadium monochloride. Further, it serves as a reagent and a strong oxidizing agent in organic synthesis.
Air & Water Reactions
Fumes in air. Reacts with moist air to form vanadic acid and hydrochloric acid fumes [Von Schwartz 1918. p.321]. Reacts exothermically with water to generate acidic fumes and acidic solutions. Violently hygroscopic.
Reactivity Profile
VANADIUM(V) TRICHLORIDE OXIDE is incompatible with bases, including amines, with strong oxidizing agents, and with alcohols. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291]. Combination with sodium metal led to a violent explosion [Bretherick 5th ed., 1995].
Health Hazard
Inhalation of vapor causes irritation of nose and throat. Ingestion causes irritation of mouth and stomach. Contact with eyes or skin causes severe irritation.
Fire Hazard
Special Hazards of Combustion Products: Irritating fumes of hydrogen chloride may form in fires.
Safety Profile
Poison by ingestion. A corrosive irritant to skin, eyes, and mucous membranes. Explosive reaction with sodmm. Violently hygroscopic. Violent reaction with rubidium (at 6OoC), potassium. When heated to decomposition it emits toxic fumes of VOx and Cl-. See also VANADIUM COMPOUNDS and HYDROCHLORIC ACID.
Purification Methods
VOCl3 should be lemon yellow in colour. If it is red, it may contain VCl4 and Cl2. Fractionally distil it, and then redistil it over metallic Na, but be careful to leave some residue because the residue can become EXPLOSIVE in the presence of the metal. USE A SAFETY SHIELD and avoid contact with moisture. It readily hydrolyses to vanadic acid and HCl. Store it in a tightly closed container or in sealed ampoules under N2. [Brown & Griffitts Inorg Synth I 106 1939, Brown & Griffitts Inorg Synth IV 80 1953.]
VANADIUM(V) TRICHLORIDE OXIDE Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Polygel Industries Pvt. Ltd. | 91-9819976506 | Maharashtra, India | 13 | 58 | Inquiry |
| Innovative | 07942565925 | Mumbai, India | 617 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21667 | 55 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 8673 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 57511 | 58 | Inquiry |
| ABCR GmbH & CO. KG | 49 721 95061 0 | Germany | 6846 | 75 | Inquiry |
| Wuhan Lvjing Fenghua Biotechnology Co., Ltd. | 19972070065 | China | 6436 | 58 | Inquiry |
| Nanjing Yili Biotechnology Co., Ltd. | 15251862682 | China | 8142 | 58 | Inquiry |
| Shanghai ChuCheng Trade Co., Ltd. | 021-5983532 15201998618 | China | 4619 | 58 | Inquiry |
7727-18-6(VANADIUM(V) TRICHLORIDE OXIDE)Related Search:
1of4
chevron_right




