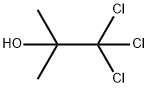
Chlorobutanol
- CAS No.
- 57-15-8
- Chemical Name:
- Chlorobutanol
- Synonyms
- HCP;Chlorbutanol;CHLORETONE;1,1,1-TRICHLORO-2-METHYL-2-PROPANOL;Methaform;2-Propanol, 1,1,1-trichloro-2-methyl-;HRC;HCPH;PTN6;PTPN6
- CBNumber:
- CB8715683
- Molecular Formula:
- C4H7Cl3O
- Molecular Weight:
- 177.46
- MOL File:
- 57-15-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | ~78 °C |
|---|---|
| Boiling point | 173 °C |
| Density | 1.3170 (estimate) |
| Flash point | >110°C |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Slightly soluble in water, very soluble in ethanol (96 per cent), soluble in glycerol (85 per cent). |
| form | Solid |
| pka | 12.87±0.29(Predicted) |
| color | Hydrated crystals |
| Odor | camphor odor |
| biological source | rabbit |
| Water Solubility | 8g/L(20 ºC) |
| Specific Activity | 820-1110nmol/min·mg |
| Stability | Stable. Generates toxic fumes on combustion. |
| LogP | 2.030 |
| CAS DataBase Reference | 57-15-8(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-(Trichloromethyl)-2-propanol(57-15-8) |
| EPA Substance Registry System | 1,1,1-Trichloro-2-methyl-2-propanol (57-15-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 |
| Hazard Codes | Xn |
| Risk Statements | 22-36/37/38 |
| Safety Statements | 26-36/37/39 |
| WGK Germany | 3 |
| Hazardous Substances Data | 57-15-8(Hazardous Substances Data) |
| Toxicity | MLD orally in dogs, rabbits (mg/kg): 238, 213 (Impens) |
Chlorobutanol Chemical Properties,Uses,Production
Chemical Properties
White or almost white, crystalline powder or colourless crystals, sublimes readily
Uses
Chlorobutanol is used as a preservative and sedative. It has also been shown to exhibit antibacterial properties.
Production Methods
Chlorobutanol is prepared by condensing acetone and chloroform in the presence of solid potassium hydroxide.
Hazard
Action similar to chloral hydrate. Combustible.
Pharmaceutical Applications
Chlorobutanol is primarily used in ophthalmic or parenteral dosage forms as an antimicrobial preservative at concentrations up to 0.5% w/v. It is commonly used as an antibacterial agent for epinephrine solutions, posterior pituitary extract solutions, and ophthalmic preparations intended for the treatment of miosis. It is especially useful as an antibacterial agent in nonaqueous formulations. Chlorobutanol is also used as a preservative in cosmetics ; as a plasticizer for cellulose esters and ethers; and has been used therapeutically as a mild sedative and local analgesic in dentistry.
Safety Profile
Poison by ingestion. A narcotic. A skin and eye irritant. Mutation data reported. See also CHLORAL HYDRATE, whch acts similarly. Dangerous; can react with oxidizing materials. Combustible when exposed to heat or flame. When heated to decomposition it emits toxic fumes of Cl-. See also PHOSGENE.
Safety
Chlorobutanol is widely used as a preservative in a number of
pharmaceutical formulations, particularly ophthalmic preparations.
Although animal studies have suggested that chlorobutanol
may be harmful to the eye, in practice the widespread use of
chlorobutanol as a preservative in ophthalmic preparations has
been associated with few reports of adverse reactions. A study of the
irritation potential of a local anesthetic on the murine cornea
indicated significant corneal surface damage in the presence of
0.5% w/v chlorobutanol, which may be related to the preservative’s
effective concentration. Reported adverse reactions to chlorobutanol
include: cardiovascular effects following intravenous
administration of heparin sodium injection preserved with chlorobutanol; neurological effects following administration of a
large dose of morphine infusion preserved with chlorobutanol;
and hypersensitivity reactions, although these are regarded as
rare.
The lethal human dose of chlorobutanol is estimated to be
50–500 mg/kg.
LD50 (dog, oral): 0.24 g/kg
LD50 (mouse, oral): 0.99 g/kg
LD50 (rabbit, oral): 0.21 g/kg
storage
Chlorobutanol is volatile and readily sublimes. In aqueous solution,
degradation to carbon monoxide, acetone and chloride ion is
catalyzed by hydroxide ions. Stability is good at pH 3 but becomes
progressively worse with increasing pH. The half-life at pH 7.5
for a chlorobutanol solution stored at 258℃ was determined to be
approximately 3 months. In a 0.5% w/v aqueous chlorobutanol
solution at room temperature, chlorobutanol is almost saturated
and may crystallize out of solution if the temperature is reduced.
Losses of chlorobutanol also occur owing to its volatility, with
appreciable amounts being lost during autoclaving; at pH 5 about
30% of chlorobutanol is lost. Porous containers result in losses
from solutions, and polyethylene containers result in rapid loss.
Losses of chlorobutanol during autoclaving in polyethylene
containers may be reduced by pre-autoclaving the containers in a
solution of chlorobutanol; the containers should then be used
immediately. There is also appreciable loss of chlorobutanol
through stoppers in parenteral vials.
The bulk material should be stored in an airtight container at a
temperature of 8–158℃.
Incompatibilities
Owing to problems associated with sorption, chlorobutanol is incompatible with plastic vials, rubber stoppers, bentonite, magnesium trisilicate, polyethylene, and polyhydroxyethylmethacrylate, which has been used in soft contact lenses. To a lesser extent, carboxymethylcellulose and polysorbate 80 reduce antimicrobial activity by sorption or complex formation.
Regulatory Status
Included in the FDA Inactive Ingredients Database (IM, IV, and SC
injection; inhalations; nasal, otic, ophthalmic, and topical preparations).
Labeling must state ‘contains chlorobutanol up to 0.5%.’
Included in nonparenteral and parenteral medicines licensed in the
UK. Included in the Canadian List of Acceptable Non-medicinal
Ingredients.
In the UK, the maximum concentration of chlorobutanol
permitted for use in cosmetics, other than foams, is 0.5%. It is
not suitable for use in aerosols.
Chlorobutanol Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SYNOVA CHEMICALS | +91-9920741772 +91-9920741772 | Mumbai, India | 428 | 58 | Inquiry |
| Atul Bioscience Ltd | +91-9316723499 +91-9316723499 | Maharashtra, India | 43 | 58 | Inquiry |
| Laxachem Organics Pvt Ltd | +91-9370153672 +91-9820138604 | Maharashtra, India | 9 | 58 | Inquiry |
| Vijaya Pharma And Life Science | +91-8939866271 +91-8939866271 | Tamil Nadu, India | 320 | 58 | Inquiry |
| Laxachem Organics Pvt. Ltd | +91-22-2343 8127 | New Delhi, India | 13 | 30 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6700 | 30 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| Milan Chemicals | 08048372496Ext 251 | Gujarat, India | 2 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| SYNOVA CHEMICALS | 58 |
| Atul Bioscience Ltd | 58 |
| Laxachem Organics Pvt Ltd | 58 |
| Vijaya Pharma And Life Science | 58 |
| Laxachem Organics Pvt. Ltd | 30 |
| CHEMSWORTH | 30 |
| CLEARSYNTH LABS LTD. | 58 |
| A.J Chemicals | 58 |
| Pharmaffiliates Analytics and Synthetics P. Ltd | 58 |
| Milan Chemicals | 58 |
57-15-8(Chlorobutanol)Related Search:
1of4
chevron_right




