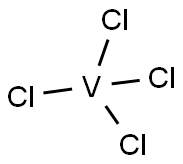
VANADIUM (IV) CHLORIDE
- CAS No.
- 7632-51-1
- Chemical Name:
- VANADIUM (IV) CHLORIDE
- Synonyms
- VCl4;VANADIUM CHLORIDE;Tetrachlorovanadium;vanadiumchloride(vcl4);Vanadium tetrachloride;VANADIUM (IV) CHLORIDE;Tetrachlorovanadium(IV);Vanadium(IV) tetrachloride;VANADIUM (IV) CHLORIDE 99+%;(t-4)-vanadiumchloride(vcl4
- CBNumber:
- CB7328482
- Molecular Formula:
- Cl4V
- Molecular Weight:
- 192.75
- MOL File:
- 7632-51-1.mol
- Modify Date:
- 2024/7/12 14:58:14
| Melting point | −28 °C(lit.) |
|---|---|
| Boiling point | 154 °C(lit.) |
| Density | 1.816 g/mL at 25 °C(lit.) |
| vapor pressure | 10.173hPa at 25℃ |
| Flash point | 148.5°C/755mm |
| storage temp. | water-free area |
| solubility | diethyl ether: slightly soluble(lit.) |
| form | Liquid |
| color | Opaque reddish-brown |
| Water Solubility | soluble alcohol, ether [HAW93] |
| Sensitive | Moisture Sensitive |
| Dielectric constant | 3.1(Ambient) |
| LogP | 0 at 20℃ |
| EPA Substance Registry System | Vanadium chloride (VCl4), (T-4)- (7632-51-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301+H311+H331-H314 | |||||||||
| Precautionary statements | P261-P280-P301+P310-P305+P351+P338-P310 | |||||||||
| Hazard Codes | T | |||||||||
| Risk Statements | 14-23/24/25-34 | |||||||||
| Safety Statements | 26-27-36/37/39-45 | |||||||||
| RIDADR | UN 2444 8/PG 1 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | YW2625000 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 28273990 | |||||||||
| NFPA 704 |
|
VANADIUM (IV) CHLORIDE Chemical Properties,Uses,Production
Chemical Properties
Red liquid. Decomposes slowly to vanadium trichloride and chlorine below63C. Soluble in absolute alcohol and ether. Nonflammable.
Uses
Preparation of vanadium trichloride, vanadium dichloride, and organovanadium compounds.
Preparation
Disproportionate of VCl3 to VCl2 and VCl4 by heating in a N2 stream at 900°C in a porcelain tube.
Air & Water Reactions
Forms acid mists in moist air. Reacts with water to form corrosive fumes of HCl. Handling Chemicals Safely 1980. p. 952).
Reactivity Profile
VANADIUM (IV) CHLORIDE decomposes by the action of light with the formation of Cl2 gas. Reacts as an acid to neutralize bases. These neutralizations generate heat. Usually does not react as either oxidizing agents or reducing agents but such behavior is not impossible. May catalyze organic reactions.
Health Hazard
CORROSIVE and/or TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire will produce irritating, corrosive and/or toxic gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Contact with molten substance may cause severe burns to skin and eyes. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
EXCEPT FOR ACETIC ANHYDRIDE (UN1715), THAT IS FLAMMABLE, some of these materials may burn, but none ignite readily. May ignite combustibles (wood, paper, oil, clothing, etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Flammable/toxic gases may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water. Substance may be transported in a molten form.
Safety Profile
Poison by ingestion. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of VOx and Cl-. See also VANADIUM COMPOUNDS and HYDROCHLORIC ACID.
Potential Exposure
Vanadium tetrachloride is used as a fixative in textile dyeing and in the manufacture of other vanadium compounds.
Shipping
UN2444 Vanadium tetrachloride, Hazard class: 8; Labels: 8-Corrosive material.
Incompatibilities
Vanadium tetrachloride is chemically unstable, highly reactive with all forms of moisture, including humidity, releasing corrosive hydrogen chloride and/or chloride fumes. Keep away from light, UV, water, steam, lithium, chlorine, trifluoride, alcohols, organic, and combustible materials. Vanadium tetrachloride is a reactive chemical and is an explosion hazard. See storage and handling section. Corrosive to metals and may release flammable hydrogen gas. Vanadium tetrachloride decomposes by the action of light with the formation of Cl2 gas. Reacts as an acid to neutralize bases. These neutralizations generate heat. Usually does not react as either oxidizing agents or reducing agents but such behavior is not impossible. May catalyze organic reactions.
VANADIUM (IV) CHLORIDE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21670 | 55 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Fuxin Pharmaceutical | +86-021-021-50872116 +8613122107989 | China | 10297 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
| Zhuoer Chemical Co., Ltd | 02120970332; +8613524231522 | China | 3005 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 9003 | 58 | Inquiry |
| LEAPCHEM CO., LTD. | +86-852-30606658 | China | 43348 | 58 | Inquiry |
| Shanghai Likang New Materials Co., Limited | +86-16631818819 +86-17736933208 | China | 9311 | 58 | Inquiry |
| ABCR GmbH & CO. KG | 49 721 95061 0 | Germany | 6846 | 75 | Inquiry |





