ChemicalBook > Product Catalog >Inorganic chemistry >Inorganic salts >Metal halide and Halogen salt >Metal chlorides and salt >VANADIUM(II) CHLORIDE
VANADIUM(II) CHLORIDE
- CAS No.
- 10580-52-6
- Chemical Name:
- VANADIUM(II) CHLORIDE
- Synonyms
- dichlorovanadium;VANADIUM DICHLORIDE;VANADIUM(II) CHLORIDE;Vanadium(II) dichloride;VANADIUM(II) CHLORIDE 95%;Vanadium(II) Chloride Powder;Vanadium chloride(VCl2) (6CI,8CI,9CI)
- CBNumber:
- CB6123952
- Molecular Formula:
- Cl2V
- Molecular Weight:
- 121.85
- MOL File:
- 10580-52-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/12 9:00:28
| Melting point | 910(subl.) |
|---|---|
| Boiling point | 910°C |
| Density | 3.09 g/mL at 25 °C(lit.) |
| solubility | reacts with H2O; soluble in ethanol,ethyl ether |
| form | green hexagonal plates |
| color | green hexagonal, hexane plates |
| Water Solubility | decomposed in hot H2O; soluble alcohol, ether [HAW93] |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|---|---|
| Signal word | Danger |
| Hazard statements | H302-H314 |
| Precautionary statements | P280-P305+P351+P338-P310 |
| Hazard Codes | C |
| Risk Statements | 20/21/22-34-22 |
| Safety Statements | 26-27-36/37/39-45 |
| RIDADR | UN 3260 8/PG 2 |
| WGK Germany | 3 |
| RTECS | YW1575000 |
| F | 3-10 |
| HazardClass | 8 |
| PackingGroup | III |
| HS Code | 2827398590 |
VANADIUM(II) CHLORIDE price More Price(2)
VANADIUM(II) CHLORIDE Chemical Properties,Uses,Production
Chemical Properties
apple green; hexagonal plates; strong reducing agent; preparation: heating VCl3 in N2 atmospheric, followed by sublimation in N2 atmospheric; used to purify HCl by removing arsenic [HAW93]
Synthesis
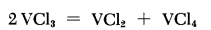
Rapid disproportionation of VCl3 according to the above equation
can be achieved at 800°C in a stream of high-purity N2. The VCl4 is carried away by the N2 stream (4 bubbles/sec), while the VCl2
remains in the reactor tube. The temperature should not exceed
850°C, to avoid loss of VCl2 by sublimation. The reaction is fairly
fast; for example, the reaction of 20 g. of VCl3 takes 2 hours.
Solubility in organics
Insoluble in alcohol or ether.
VANADIUM(II) CHLORIDE Preparation Products And Raw materials
Raw materials
Preparation Products
Global( 34)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Alfa Chemistry | +1-5166625404 | United States | 21317 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 9003 | 58 | Inquiry |
| Shanghai Acmec Biochemical Technology Co., Ltd. | +undefined18621343501 | China | 33350 | 58 | Inquiry |
| Shanghai Haohong Scientific Co., Ltd. | 400-400-8210725 | China | 40041 | 58 | Inquiry |
| Shanghai Macklin Biochemical Co.,Ltd. | 15221275939 15221275939 | China | 16168 | 55 | Inquiry |
| Jiangsu Aikon Biopharmaceutical R&D co.,Ltd. | 025-66113011 18112977050 | China | 10529 | 58 | Inquiry |
| Shanghai Guchen Biotechnology Co., LTD | 021-34675735 19147740836 | China | 9849 | 58 | Inquiry |
| Guangdong Fangxin Biotechnology Co., Ltd. | 0751-2838688 13376594225 | China | 9937 | 58 | Inquiry |
| Shandong Xiya Chemical Co., Ltd. | 4009903999 13395398332 | China | 20810 | 60 | Inquiry |
| Shanghai Yingxin laboratory equipment Co., Ltd | 021-021-59178156 15300768757 | China | 10678 | 58 | Inquiry |
10580-52-6(VANADIUM(II) CHLORIDE)Related Search:
VANADIUM DICHLORIDE
VANADIUM(II) CHLORIDE
VANADIUM(II) CHLORIDE 95%
Vanadium(II) dichloride
Vanadium chloride(VCl2) (6CI,8CI,9CI)
dichlorovanadium
Vanadium(II) Chloride Powder
10580-52-6
Cl2V
VCl2
Vanadium
Catalysis and Inorganic Chemistry
Catalysis and Inorganic Chemistry
Chemical Synthesis
Salts
Vanadium Salts
VanadiumMetal and Ceramic Science





