EFONIDIPINE
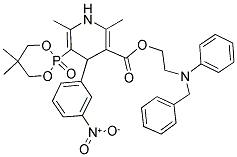
- CAS No.
- 111011-76-8
- Chemical Name:
- EFONIDIPINE
- Synonyms
- nz-105;nz105ethanolate;Hydrochloride ethanol;EFONIDIPINE USP/EP/BP;r,p-oxide,monohydrochloride;efonidipinehydrochlorideethanolate;NZ-105 HYDROCHLORIDE MONOETHANOLATE;2-(phenyl(phenylmethyl)amino)ethyleste;Efonidipine hydrochloride monoethanolate;Efonidipine Hydrochloride ethanol (1:1:1)
- CBNumber:
- CB9317820
- Molecular Formula:
- C34H38N3O7P
- Molecular Weight:
- 631.66
- MOL File:
- 111011-76-8.mol
- Modify Date:
- 2024/7/2 8:55:41
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Warning |
| Hazard statements | H361 |
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501 |
EFONIDIPINE price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | E0159 | Efonidipine hydrochloride monoethanolate ≥98% (HPLC) | 111011-76-8 | 5MG | ₹9255.38 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | E0159 | Efonidipine hydrochloride monoethanolate ≥98% (HPLC) | 111011-76-8 | 25MG | ₹36415.3 | 2022-06-14 | Buy |
EFONIDIPINE Chemical Properties,Uses,Production
Description
Efonidipine hydrochloride ethanol was first launched in Japan for the treatment of essential severe and renal hypertension. As the fourteenth dihydropyridine (DHP) calcium channel blocker to reach the market, it functions by binding to the DHP receptors on voltage-dependent calcium channels to cause diuretic and natriuretic actions in patients with essential hypertension. Its vasodilating and calcium antagonist effects are very slow in onset and long-lasting in all types of experimentally hypertensive models and the agent is reported to exhibit an improved side effect profile compared with other drugs of the same class. This may be explained by the slow and long-lasting inhibition of transmembrane Ca+2 uptake, which is accompanied by its very slow binding to and dissociation from the DHP receptors. A recent study in cholesterol-fed rabbits suggested that efonidipine may suppress the development of atherosclerosis without affecting the plasma lipids. Clinical trials for angina, peetoris, and for cerebrovasodilatory disorders have been reported.
Uses
Efonidipine Hydrochloride Monoethanolate is a potent inhibitor of the L-type calcium channel.
Biological Activity
Selective blocker of L-type and T-type Ca 2+ channels. Displays minimal inhibition of N- and P/Q-type channels and no inhibition of R-type channels. R (-) and S (+)-enantiomers displays different channel selectivity; S (+)-Efonidipine blocks L-type and T-type channels whereas R (-)-Efonidipine displays selectivity for T-type channels. Exhibits antihypertensive activity.
EFONIDIPINE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Angle Biopharma | 08046067501 | Ahmedabad, India | 142 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6761 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21675 | 55 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 9030 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 26165 | 58 | Inquiry |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 | China | 5999 | 58 | Inquiry |
| Nantong HI-FUTURE Biology Co., Ltd. | +undefined18051384581 | China | 3136 | 58 | Inquiry |





