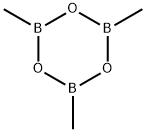
Trimethylboroxine
|
|
Trimethylboroxine 속성
- 녹는점
- −38 °C(lit.)
- 끓는 점
- 78-80 °C(lit.)
- 밀도
- 0.898 g/mL at 25 °C(lit.)
- 굴절률
- n
20/D 1.362(lit.)
- 인화점
- 16 °F
- 저장 조건
- Flammables area
- 용해도
- Chloroform (Sparingly), Dichloromethane (Slightly), THF (Soluble)
- 물리적 상태
- 액체
- 색상
- 무색~황색
- 수용성
- 물과 섞이지 않습니다.
- 감도
- Moisture Sensitive & Hygroscopic
- BRN
- 1757008
- 노출 한도
- ACGIH: TWA 50 ppm; STEL 100 ppm (Skin)
OSHA: TWA 200 ppm(590 mg/m3)
NIOSH: IDLH 2000 ppm; TWA 200 ppm(590 mg/m3); STEL 250 ppm(735 mg/m3)
- InChIKey
- GBBSAMQTQCPOBF-UHFFFAOYSA-N
- CAS 데이터베이스
- 823-96-1(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F,C,Xi | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 11-34-41-37/38-19 | ||
| 안전지침서 | 9-16-26-33-36/37/39-45-46-37/39-3 | ||
| 유엔번호(UN No.) | UN 2924 3/PG 2 | ||
| WGK 독일 | 3 | ||
| F 고인화성물질 | 2-10-21 | ||
| TSCA | Yes | ||
| 위험 등급 | 3.1 | ||
| 포장분류 | II | ||
| HS 번호 | 29319090 |
Trimethylboroxine C화학적 특성, 용도, 생산
화학적 성질
colorless to yellow solution용도
Trimethylboroxine is used as a derivatizing agent for GLC analysis. It is used in a diverse array of areas, including as a polymerization additive. It is also used in the preparation of CBS catalysts for asymmetric reductions.제조 방법
Trimethylboroxine is prepared by heating B(CH3)3 and B2O3 together in a sealed tube. The B2O3 powder is made by dehydrating H3BO3 under vacuum over P2O5 , at 220℃. The very hygro-scopic oxide is placed with strict exclusion of moisture in a 200-ml. thick-wall Pyrex tube provided with a ground joint, and a melting-point capillary is fastened to the tube just below the joint. The tube is connected to a vacuum pump and immersed in liquid nitrogen, and when a high vacuum has been established, a quantity of B(CH3)3 equivalent to 4.25 g. (0.061 mole) of B2O3 is condensed in the tube. The tube is sealed off, heated to 600℃ and kept at this temperature for six hours; in the process the contents turn into a clear, colorless liquid. When the tube has cooled down, the tip is broken under a nitrogen blanket and sealed to a tube leading to the vacuum pump. The tube is evacuated and the contents of the tube are transferred into a -78℃ trap. The crude product is purified by removing volatile contaminants at -45℃ and then distilling the product from a -10℃ trap into a receiver held at -78℃.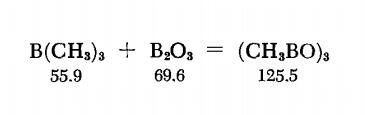
주요 응용
Trimethylboroxine (TMB) is a cyclic anhydride of methyl-boronic acid. It can be been used:As a derivatizing agent for gas chromatographic/mass spectrometric analysis.
In the preparation of methylaluminoxane (MAO) which is used in the polymerization of olefins.
As an electrolyte additive to enhance the interface stability of electrode/electrolyte.
In methylation of aryl halides by palladium-catalyzed Suzuki-Miyaura coupling reaction.
In the preparation of CBS (Corey, Bakshi and Shibata) catalysts for asymmetric reductions of ketones to alcohols.
Purification Methods
Possible impurity is methylboronic acid. If present, then add a few drops of conc H2SO4 and distil it immediately, then fractionate it through an efficient column. [McCusker et al. J Am Chem Soc 79 5179 1957, IR: Goubeau & Keller Z Anorg Allgem Chem 272 303 1953, Beilstein 4 IV 4378.]Trimethylboroxine 준비 용품 및 원자재
원자재
준비 용품
Trimethylboroxine 공급 업체
글로벌( 204)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Yanxi Chemical Co., Ltd. | +86-17531190177; +8617531190177 |
peter@yan-xi.com | China | 5873 | 58 |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-17333973358 |
sales06@hbduling.cn | China | 8079 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20294 | 58 |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 |
sales@capotchem.com | China | 29798 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21668 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 |
product@chemlin.com.cn | CHINA | 3012 | 60 |
| Zjartschem | +86-571 87238903 |
jocelynpan@zjarts.com | CHINA | 993 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29894 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39916 | 58 |
Trimethylboroxine 관련 검색:
헥사메틸다이실라잔 N-메틸-2-피롤리디논 메틸 실세스퀴옥산
TRIMETHYLSILYL ISOCYANATE
TriMethyl(broModifluoroMethyl)silane
Pivalic acid
Octamethylcyclotetrasilazane
METHYL-2-PYRROLIDONE
2,4,6-TRIVINYLCYCLOTRIBOROXANE PYRIDINE COMPLEX
2,4,6-TRIS(4-FLUOROPHENYL)BOROXIN
2,4,6-Tris(3,4-difluorophenyl)boroxin
TRIPHENYLBOROXIN
TRIMETHYLBORON
2,4,6-TRIS(3,4,5-TRIFLUOROPHENYL)BOROXIN
Vinylboronic anhydride pyridine complex
2,4,6-TRIS(3,5-DIMETHYLPHENYL)BOROXIN
2,4,6-Tris(3,4-dichlorophenyl)boroxin
2,4,6-TRIS(M-TERPHENYL-5'-YL)BOROXIN