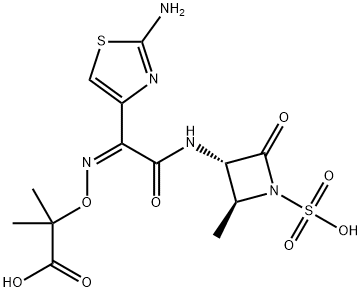
아조트리오남
|
|
아조트리오남 속성
- 녹는점
- 227°C
- 밀도
- 1.83
- 굴절률
- 1.6460 (estimate)
- 저장 조건
- room temp
- 용해도
- DMSO(약간 용해됨), 물(약간 용해됨, 가열, 초음파 처리)
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 산도 계수 (pKa)
- pKa -0.7(H2O t=RT Iunde?ned) (Uncertain);2.75(H3O t=RT Iunde?ned) (Uncertain);3.91(H4O t=RT Iunde?ned) (Uncertain)
- 색상
- 흰색에서 베이지색
- 수용성
- 50mg/ml에서 DMF/물(1:1)에 용해됨
- Merck
- 14,925
- InChIKey
- WZPBZJONDBGPKJ-VEHQQRBSSA-N
- SMILES
- C(O)(=O)C(O/N=C(/C1=CSC(N)=N1)\C(N[C@@H]1C(=O)N(S(O)(=O)=O)[C@H]1C)=O)(C)C
- CAS 데이터베이스
- 78110-38-0(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | Xn | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 20/21/22-36/37/38 | ||
| 안전지침서 | 22-24/25-36-26 | ||
| WGK 독일 | 2 | ||
| RTECS 번호 | UA2451400 | ||
| HS 번호 | 29419000 | ||
| 독성 | TDLo ivn-rat: 1100 mg/kg (7-17D preg):TER NKRZAZ33(Suppl 1),203,85 |
| 그림문자(GHS): |
 
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Danger | |||||||||||||||||||||
| 유해·위험 문구: |
|
|||||||||||||||||||||
| 예방조치문구: |
|
아조트리오남 C화학적 특성, 용도, 생산
개요
Aztreonam is the first member of the monobactam class of antibiotics to be introduced into the world market. It possesses high β-lactamase stability and moderately good activity against gram negative aerobes such as E. coli, S. marcescens, -9 Proteus Providencia, Salmonella, g. influenzae, E. gonorrhea, and &. pneumonia. While somewhat less potent against Pseudomonas aeruginosa, it is nonetheless one of the better β-lactams against this species. It has poor activity against gram positive organisms.화학적 성질
White Crystalline Powder역사
Aztreonam was synthesized by the Squibb Institute for Medical Research in 1981 starting with l-threonine. The synthesis was based on findings about bacterial β-lactam compounds of a monocyclic nature . The β-lactam compounds, called monobactams, were isolated from Chromobacterium violaceum, Agrobacterium radiobacter, etc. Such monocyclic βlactams of bacterial origin had previously been found independently in 1981 by Takeda Chemicals Industries in the culture broths of Pseudomonas acidophila and P. mesoacidophila and named sulfazecin and isosulfazecin, respectively. Aztreonam was selected from among hundreds of derivatives as a candidate for clinical trials because of its unique antibacterial spectrum and strong activity. This antibiotic shows excellent activity against a variety of gram-negative aerobic bacteria but no activity against gram-positive bacteria or anaerobes. Its efficacy and safety are now being clinically evaluated.용도
Aztreonam is a synthetic β-lactam antibiotic of the monobactam class. It is effective against Gram-negative bacteria but inactive against Gram-positive bacteria. Its mechanism of action involves the inhibition of mucopeptide synthesis in the bacterial cell wall, thereby blocking peptidoglycan crosslinking. Aztreonam is resistant to hydrolysis by some β-lactamases, but is inactivated by extended-spectrum β-lactamases.Antimicrobial activity
Concentrations (mg/L) inhibiting 50% of other organisms are: Aeromonas spp., 0.1;Acinetobacter spp., 16; Mor. catarrhalis, 0.1; Burkholderia cepacia, 2; and Yersinia spp., 0.1. Synergy has been shown with gentamicin, tobramycin and amikacin against 52–89% of strains of Ps. aeruginosa and gentamicin-resistant Gram-negative bacteria.Pharmacokinetics
Cmax 1 g intravenous: 90 mg/L end infusion1 g intramuscular: 46 mg/L after 1 h
Plasma half-life: 1.7 h
Volume of distribution: 0.18 L/kg
Plasma protein binding: 56%
Absorption and distribution
Oral bioavailability is less than 1%. Peak concentrations above the median MIC for most Gram-negative pathogens are achieved in most tissues and body fluids after 1 g intramuscular or intravenous doses.
Metabolism and excretion
It is not extensively metabolized, the most prominent product, resulting from opening the β-lactam ring, being scarcely detectable in the serum and accounting for about 6% of the dose in the urine and 3% in the feces.
It is predominantly eliminated in the urine, where 58–72% appears within 8 h. Less than 12% is eliminated unchanged in the feces, suggesting low biliary excretion.
Clinical Use
Urinary tract infections, including pyelonephritis and cystitisLower respiratory tract infections, including pneumonia and bronchitis
caused by Gram-negative bacilli
Septicemia
Skin and skin structure infections, including postoperative wounds, ulcers
and burns
Intra-abdominal infections, including peritonitis
Gynecological infections, including endometritis and pelvic cellulitis
부작용
Local reactions occasionally occur at the injection site. Systemic reactions include diarrhea, nausea and/or vomiting and rash (1–1.3%). Neutropenia was seen in 11.3% of the pediatric patients younger than 2 years. Pseudomembranous colitis has been reported.There are no reactions in patients with immunoglobulin E (IgE) antibodies to benzylpenicillin or penicillin moieties. It is rarely cross-reactive with other β-lactam antibiotics and is weakly immunogenic.
Safety Profile
Moderately toxic by severalroutes. An experimental teratogen. Other experimentalreproductive effects. When heated to decomposition itemits toxic fumes of NOx and SOx.아조트리오남 준비 용품 및 원자재
원자재
이탄산 칼륨
Azetidine
L-(-)-트레오닌
메탄설포닐 클로라이드
Thiazole
벤질 클로로탄소산
글루코즈
CARBOHYDRATES
(2S-trans)-3-Amino-2-methyl-4-oxoazetidine-1-sulphonic acid
Propanoic acid, 2-[[(Z)-[1-(2-amino-4-thiazolyl)-2-[[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]amino]-2-oxoethylidene]amino]oxy]-2-methyl-, 1-(1,1-dimethylethyl) ester
2-메르캅토벤조티아졸릴-(Z)-(2-아미노티아졸-4-일)-2-(tert-부톡시카르보닐)이소프로폭시이미노아세테이트
물
준비 용품
아조트리오남 공급 업체
글로벌( 589)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Mujin Biotechnology Co.,Ltd | +86-13288715578 +86-13288715578 |
sales@hbmojin.com | China | 12747 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 |
mina@chuanghaibio.com | China | 18126 | 58 |
| Hangzhou Hyper Chemicals Limited | +86-0086-57187702781 +8613675893055 |
info@hyper-chem.com | China | 295 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-17396673057 |
linda@tnjone.com | China | 1143 | 58 |
| Shaanxi Xianhe Biotech Co., Ltd | +8617709210191 |
Jerry@xhobio.com | China | 885 | 58 |
| Capot Chemical Co.,Ltd. | +8613336195806 |
sales@capot.com | China | 29731 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21622 | 55 |
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 |
product@chemlin.com.cn | CHINA | 3009 | 60 |
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 |
zheyansh@163.com | CHINA | 3619 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29855 | 58 |