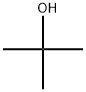
tert-부틸알코올 C화학적 특성, 용도, 생산
물리적 성질
tert-Butanol is a colorless liquid or crystals with a camphor-like odor. A detection odor threshold concentration of 2,900 mg/m3 (957 ppmv) was experimentally determined by Dravnieks (1974). In a later study, Nagata and Takeuchi (1990) reported an odor threshold concentration 220 ppbv.
용도
tert-Butanol can be used as a solvent (e.g., for paints, lacquers, and varnishes); as a denaturant for ethanol and several other alcohols; as an octane booster in gasoline; as a dehydrating agent; as a chemical intermediate in the manufacturing of methyl methacrylate; and in the manufacturing of flotation agents, fruit essences, and perfumes.
생산 방법
tert-Butanol is produced as a by-product from the isobutane
oxidation process for manufacturing propylene
oxide. It is also produced by the acidcatalyzed
hydration of isobutylene, a process no longer used
in the United States.
정의
Tert-butanol is a tertiary alcohol alcohol that is isobutane substituted by a hydroxy group at position 2. It has a role as a human xenobiotic metabolite. It derives from a hydride of an isobutane.
일반 설명
Colorless oily liquid with a sharp alcohol odor. Floats and mixes with water. Produces irritating vapor. Freezing point is 78°F.
공기와 물의 반응
Highly flammable. Water soluble.
반응 프로필
Attacks plastics. [Handling Chemicals Safely 1980. p. 236]. Acetyl bromide reacts violently with alcohols or water [Merck 11th ed. 1989]. Mixtures of alcohols with concentrated sulfuric acid and strong hydrogen peroxide can cause explosions. Example: an explosion will occur if dimethylbenzylcarbinol is added to 90% hydrogen peroxide then acidified with concentrated sulfuric acid. Mixtures of ethyl alcohol with concentrated hydrogen peroxide form powerful explosives. Mixtures of hydrogen peroxide and 1-phenylm-2-methyl propyl alcohol tend to explode if acidified with 70% sulfuric acid [Chem. Eng. News 45(43):73 1967; J, Org. Chem. 28:1893 1963]. Alkyl hypochlorites are violently explosive. They are readily obtained by reacting hypochlorous acid and alcohols either in aqueous solution or mixed aqueous-carbon tetrachloride solutions. Chlorine plus alcohols would similarly yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites [NFPA 491 M 1991]. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence [Wischmeyer 1969].
위험도
Irritant to eyes and skin. Flammable, dan-
gerous fire risk. Central nervous system impair-
ment. Questionable carcinogen.
건강위험
tert-Butanol is more toxic than secbutylalcohol but less toxic than the primaryalcohol. However, its narcotic actionis stronger than that of n-butanol. Inhalationmay cause drowsiness and mild irritationof the skin and eyes. Ingestion may produceheadache, dizziness, and dry skin.Acute oral LD50 value (rats): 3500 mg/kg.
화학 반응
Reactivity with Water: No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Moderately toxic by
ingestion, intravenous, and intraperitoneal
routes. An experimental teratogen. Other
experimental reproductive effects.
Dangerous fire hazard when exposed to heat
or flame. Moderately explosive in the form
of vapor when exposed to flame. Ignites on
contact with potassium-sodum alloys. To
fight fire, use alcohol foam, CO2, dry
chemical. Incompatible with oxidizing
materials, H202. See also n-BUTYL
ALCOHOL and ALCOHOLS.
잠재적 노출
tert-Butanol is used as solvents for
paints, lacquers, varnishes, natural and synthetic resins,
gums, vegetable oils, dyes, camphor, and alkaloids. They
are also used as an intermediate in the manufacture of pharmaceuticals and chemicals; In the manufacture of artificial
leather, safety glass; rubber and plastic cements, shellac,
raincoats, photographic films, perfumes; and in plastic
fabrication.
Carcinogenicity
There were increased incidences
of renal tubule adenoma and carcinoma in male rats,
transitional epithelia hyperplasia of the kidney in male
and female rats, follicular cell adenoma of the thyroid in
female mice, and follicular cell hyperplasia of the thyroid
and inflammation and hyperplasia of the urinary bladder in
male and female mice. In addition, a slight increase in
follicular cell adenoma or carcinoma of the thyroid (combined)
in male mice may have been related to exposure to
t-butyl alcohol.
t-Butyl alcohol was inactive on mouse skin as a complete
carcinogen or as a tumor promoter.
운송 방법
UN1120 Butanols, Hazard Class: 3; Labels: 3—
Flammable liquid. UN1212 Isobutanol or Isobutyl alcohol,
Hazard Class: 3; Labels: 3—Flammable liquid
Purification Methods
tert-Butanol is synthesised commercially by the hydration of 2-methylpropene in dilute H2SO4. Dry it with CaO, K2CO3, CaSO4 or MgSO4, filter and fractionally distil it. Dry further by refluxing with, and distilling from, either magnesium activated with iodine, or small amounts of calcium, sodium or potassium, under nitrogen. Passage through a column of type 4A molecular sieve is another effective method of drying; as well as refluxing with tert-butyl phthalate or succinate. (For method see Ethanol.) Other methods include refluxing with excess aluminium tert-butylate, or standing with CaH2, and distilling as needed. Further purification is achieved by fractional crystallisation by partial freezing, taking care to exclude moisture. tert-Butyl alcohol samples containing much water can be dried by adding *benzene, so that the water distils off as a tertiary azeotrope, b 67.3o. Traces of isobutylene have been removed from dry tert-butyl alcohol by bubbling dry pre-purified nitrogen through for several hours at 40-50o before using. It forms azeotropic mixtures with a large number of compounds. It has also been purified by distillation from CaH2 into Linde 4A molecular sieves which had been activated at 350o for 24hours [Jaeger et al. J Am Chem Soc 101 717 1979]. [Beilstein 1 IV 1609.] Rapid purification: Dry tert-butanol over CaH2 (5% w/v), distil and store it over 3A molecular sieves.
비 호환성
tert-Butanol is incompatible with strong acids (including mineral acid), including mineral acids; strong oxidizers or caustics, aliphatic amines; isocyanates, alkali metals (i.e., lithium, sodium, potassium, rubidium, cesium, francium).
폐기물 처리
Incineration, or bury absorbed
waste in an approved land fill.
tert-부틸알코올 준비 용품 및 원자재
원자재
준비 용품