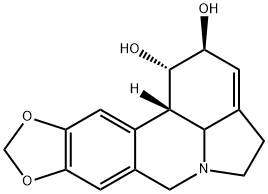
LYCORINE
|
|
LYCORINE 속성
- 녹는점
- 253-255℃ (dec.)
- 알파
- D16 -129° (c = 0.16 in 98% alc)
- 끓는 점
- 429.61°C (rough estimate)
- 밀도
- 1.53
- 굴절률
- 1.5500 (estimate)
- 저장 조건
- 2-8°C
- 용해도
- Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc.
- 산도 계수 (pKa)
- 13.55±0.40(Predicted)
- 물리적 상태
- 가루
- 색상
- White to off-white
- optical activity
- -12916 (c 0.16, 98% ethanol)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 그림문자(GHS): |

|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Danger | ||||||||||||||||
| 유해·위험 문구: |
|
||||||||||||||||
| 예방조치문구: |
|
LYCORINE C화학적 특성, 용도, 생산
개요
Lycoris radiate, a traditional Chinese medicine (TCM), is the bulb of the amaryllidaceous Lycoris radiata herb. It has been applied for clinical purposes for centuries. It is firstly recorded in Tujing Bencao and mainly used for the pyogenic infections. According to A Supplement to Compendium of Materia Medica, lycoris radiate may be used for treating acute throat trouble, phlegm node, baihuodan, and pulmonary abscess.There are about 20 Lycoris species in the world, which are widely distributed in China and Japan. Lycoris radiate is an amazing horticultural plant with a graceful shape and a bright color. In TCM, it is acrid in taste and neutral in nature, with functions of detoxication, easy expectoration, and diuresis and emesis promotion. According to the modern medicine, lycoris radiate is considered to be in favor of the central nervous system and cardiovascular system.
The main active ingredients extracted from the lycoris herbs are about 40 alkaloids with various contents. Pharmacological tests indicate that galanthamine, lycorine, lycoramine, lycorenine, and crinine are the major effective medicinal ingredients. Lycorine may be used to treat amebic dysentery and against cancer. Moreover, galanthamine, dihydrogalanthamine, and lycoramine may be used to treat infantile paralysis and restore nerve functions and against traumatic paraplegia, etc. Lycoris radiate is famous because galanthamine has been approved by FDA as an anti-Alzheimer disease drug. Lycoris radiate is the only natural source for galanthamine with extremely low content (<0.02%).
물리적 성질
Appearance: colorless prismatic crystal. Solubility: insoluble in water; sparingly soluble in ethyl alcohol and diethyl ether. Melting point: 275–280?°C (decomposition). Specific optical rotation: right-handed optical rotation with a specific optical rotation of ?129° (98% ethyl alcohol).역사
In 1895, Morishima successfully extracted lycorine from the bulb of Lycoris radiata. However, its structure was unidentified until in 1935. In 1959, its stereochemical structure was dissected by monocrystal.The solvent extraction method, chromatographic separation, and resin absorption are commonly used for lycorine extraction. However, lycorine obtained from these techniques is not pure enough and often mixed with other alkaloids. The great differences in the efficacies of different alkaloids prevent such blending from being directly used. Furthermore, the separation and purification process so required has an adverse effect on and limits the development and utilization of the medicinal value of lycorine.
용도
Lycorine is an analgesic, more so than aspirin, and a hypotensive, as are caranine and galanthine . The analgesic activity exhibited by the Amaryllidaceae alkaloids is attributed to their resemblance to the morphine and codeine skeletons. Lycorine also has antiarrhythmic action, and lycorine hydrochloride is a strong broncholytic. In fact, lycorine shows a relaxant effect on an isolated epinephrine-precontracted pulmonary artery and increases contractility and the rate of an isolated perfused heart. These effects are mediated by stimulation of b-adrenergic receptors.Lycorine also has a strong inhibitory effect on parasite (Encephalitozoon intestinalis) development and antifungal activity against Candida albicans. Additionally, lycorine has antifeedant, antimalarial, emetic, anti-inflammatory, antiplatelet , as well as antifertility activities. Galanthine, in turn, shows mild in vitro activity against Tripanosoma brucei rhodesiense and Plasmodium falciparum.
정의
ChEBI: An indolizidine alkaloid that is 3,12-didehydrogalanthan substituted by hydroxy groups at positions and 2 and a methylenedioxy group across positions 9 and 10. Isolated from Crinum asiaticum, it has been shown to exhibit antimalarial activit .Indications
Injection: 25?mg/ml, for resistance to amebic protozoa and treatment of intraintestinal/extraintestinal amebiasis. Subcutaneous injection: 25–50? mg/injection and 50?mg/day.Clinical Use
Dihydrolycorine, generated through the hydrogenation of lycorine, has been used clinically due to its better resistance against amebic dysentery and lower toxicity. The amine salt made of lycorine has an antitumor effect in animals.Lycorine exposure may cause skin irritation (red and swollen) and itching. Nosebleed may be induced in case of inhalation. In case of overdose, it may cause salivation, emesis, diarrhea, bradycardia, cold hands/feet, or even death due to respiratory center paralysis. The major studies of clinical application are focused on (1) antitumor effect, (2) effect on the central nervous system, (3) effect on the cardiovascular system, (4) anti-inflammatory effect, (5) effect on smooth muscle, and (6) emetic effect.
Purification Methods
It crystallises as orange crystals from MeOH (m 281-283o), CHCl3/EtOH (m 272-274o), pyridine or from EtOH (m 277o, dec). It has been distilled under high vacuum. The hydrochloride has m 288o (from MeOH/HCl), and the picrate has m 196-197o(from EtOH), [Cook et al. J Chem Soc 4176 1954, Martin & Tu J Org Chem 46 3763 1981, Beilstein 27 II 547, 27 III/IV 6463.]LYCORINE 준비 용품 및 원자재
원자재
준비 용품
LYCORINE 공급 업체
글로벌( 150)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Shanghai Zheyan Biotech Co., Ltd. | 18017610038 |
zheyansh@163.com | CHINA | 3619 | 58 |
| Chengdu Greenpure Biopharma CO.,Ltd | 18283602253; +8618283602253 |
jancyzheng@gcgreenpure.com | China | 953 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29878 | 58 |
| Chengdu GLP biotechnology Co Ltd | 028-87075086 13350802083 |
scglp@glp-china.com | CHINA | 1824 | 58 |
| Nanjing Dolon Biotechnology Co.,Ltd. | 18905173768 |
sales@dolonchem.com | CHINA | 2972 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39894 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49374 | 58 |
| SIMAGCHEM CORP | +86-13806087780 |
sale@simagchem.com | China | 17365 | 58 |
| Wuhan ChemNorm Biotech Co.,Ltd. | +86-27-8439 4403 18971486879 |
sales@chemnorm.com | CHINA | 2935 | 58 |