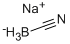나트륨 사이아노브로민하이드라이드(나트륨 시아노브롬하이드리드)
|
|
나트륨 사이아노브로민하이드라이드(나트륨 시아노브롬하이드리드) 속성
- 녹는점
- >242 °C (dec.) (lit.)
- 끓는 점
- 307°C
- 밀도
- 1.083 g/mL at 25 °C
- 인화점
- −1 °F
- 저장 조건
- Store below +30°C.
- 용해도
- 물(100mg/ml, 가열 시), 메탄올, 에탄올 및 THF에 용해됩니다. 벤젠이나 헥산과 같은 비극성 용매에는 녹지 않습니다.
- 물리적 상태
- 가루
- 색상
- 하얀색
- Specific Gravity
- 1.2
- 수용성
- 29°C(12월)에서 2120g/L
- 감도
- Moisture Sensitive
- Merck
- 14,8606
- BRN
- 4152656
- 안정성
- 안정적인. 흡습성. 물과 격렬하게 반응하여 수소를 방출하고 발화함. 이 화학물질과 관련된 화재에는 물을 사용하지 마십시오. 대신 건조한 소다회를 사용하십시오. 강산, 물, 강산화제와 호환되지 않습니다.
- CAS 데이터베이스
- 25895-60-7(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T+,N,T,F | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 26/27/28-32-34-50/53-16-15-11-51/53-36-23/24/25-19-14-40-36/37/38 | ||
| 안전지침서 | 26-28-36/37/39-45-60-61-8-50A-43-28A-1-16-36/37 | ||
| 유엔번호(UN No.) | UN 3179 4.1/PG 2 | ||
| WGK 독일 | 1 | ||
| F 고인화성물질 | 10-21 | ||
| 위험 참고 사항 | Toxic/Highly Flammable | ||
| TSCA | Yes | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | I | ||
| HS 번호 | 28500020 | ||
| 기존화학 물질 | KE-05-1186 | ||
| 유해화학물질 필터링 | 97-1-90 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 무기시안 화합물 및 이를 1% 이상 함유한 혼합물. 다만, 베를린청(Ferric ferrocyanide), 페로시안염(Ferrocyanide, salts), 페리시안염(Ferricyanide, salts) 및 그 중 하나를 함유한 혼합물은 제외 |
나트륨 사이아노브로민하이드라이드(나트륨 시아노브롬하이드리드) C화학적 특성, 용도, 생산
물성
안정적. 흡습성. 물과 격렬하게 반응하여 수소를 방출하고 점화합니다. 이 화학 물질과 관련된 화재에는 물을 사용하지 말고 대신 마른 소다회를 사용하십시오. 강산, 물, 강한 산화제와는 호환되지 않습니다.용도
사용 나트륨 시아 노 수소화 붕소는 알데하이드 및 케톤의 환원성 아미 노화 및 아민의 환원성 알킬화 시약으로 일반적으로 사용된다.개요
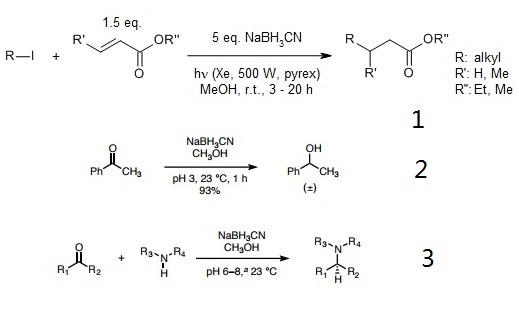
Sodium cyanoborohydride (NaBH3CN) is a selective reducing agent used for a variety of chemical reductions, including aldehyde, ketones, acetals, epoxides, oximes, enamines, reductive aminations of aldehydes and ketones, and reductive alkylations of amines and hydrazines. The utility of sodium cyanoborohydride as a reducing agent is greatly enhanced by its stability under acid conditions, and its solubility in aprotic solvents. Sodium cyanoborohydride is a milder and more selective reducing agent than sodium borohydride.Sodium cyanoborohydride is a weaker and more-selective reducing agent than sodium borohydride because of the electron-withdrawing effect of the cyano group. It has the further advantage that it is stable in acid to pH = 3 and can be employed to effect reductions in the presence of functional groups that are sensitive to the more-basic conditions of reduction with sodium borohydride.
Aldehydes and ketones are unaffected by sodium cyanoborohydride in neutral solution, but they are readily reduced to the corresponding alcohol at pH=3-4 by way of the protonated carbonyl group. By previous exchange of the hydrogens of the borohydride for deuterium or tritium, by reaction with D2O or tritiated water, an efficient and economical route is available for deuteride or tritiide reduction of aldehydes and ketones.
화학적 성질
Sodium cyanoborohydride is a white or slightly yellow solid powder, It is a versatile reducing agent stable in aqueous solution at pH 7.2. It is a weaker reducing agent as compared to NaBH4. It effectively reduces Schiff bases. NaCNBH4 participates in the reductive methylation for the efficient and selective conversion of the e-amino groups of lysyl residues in proteins to the mono- and dimethyl derivatives.물리적 성질
Sodium cyanotrihydridoborate, sodium cyanoborohydride, sodium cyanoboranate, NaBH3CN, is a white, amorphous powder, melting with decomposition at 240 – 242℃. Contact with the atmosphere must be avoided as it is extremely hygroscopic. It is very soluble in polar, especially protic, solvents such as water, alcohols, amines, and THF, but not in diethyl ether or hydrocarbons.용도
Sodium Cyanoborohydride is a commonly used as a reagent in the reductive amination of aldehydes and ketones and in the reductive alkylation of amines. It is also used in the synthesis of a novel phenolate-bridged dilanthanum(III) complex of interest as a model for metalloproteins as well as for its importance in basic and applied chemistry.제조 방법
Synthesis of Sodium cyanoborohydride(NaBH3CN): NaBH3CN was made by stirring equimolar BH3·THF (~1 M) with NaCN in THF in excellent yield.To a rapidly stirred slurry of sodium borohydride (80.2g, 2.09 mol) in THF (1000mL) in a 2-L flask is added a solution of hydrogen cyanide in THF (294g containing 58.8g of hydrogen cyanide) at 25°C. Evolution of hydrogen occurs slowly during the addition. Following the addition, the reaction mixture is stirred for 1 h at 25°C and then heated at reflux until hydrogen evolution has ceased. Filtration followed by vacuum removal of THF gives white solid sodium cyanoborohydride; yield 120g (91%).화학 반응
Sodium cyanoborohydride are frequently used for reductive aminations. Since the reaction rate for the reduction of iminium ions is much faster than for ketones or even aldehydes, the reductive amination can be carried out as a one-pot procedure by introducing the reducing agent into a mixture of the amine and carbonyl compound.Contact with strong acids liberates the highly toxic gas HCN. A safer reducing agent with comparable reactivity is sodium triacetoxyborohydride. Reduction with Sodium Cyanoborohydride:
Tin-free Giese reaction of alkyl iodides with electron-deficient alkenes and the related radical carbonylation process proceeded efficiently in the presence of sodium cyanoborohydride and tetrabutylammonium cyanoborohydride. Transfer of iodine followed by hydride reduction of the resulting carbon-iodine bond is proposed as a possible mechanism.
Borch and co-workers showed that sodium cyanoborohydride and lithium cyanoborohydride are acid-stable reagents capable of rapidly reducing carbonyl compounds to alcohols at pH 3-4, presumably via a protonated carbonyl cation.
With care to maintain a pH of 6-7, a mixture of a ketone or aldehyde reactant, an amine, and sodium cyanohydride provides products of reductive amination selectively, without competitive reduction of the carbonyl substrate. Though the conditions of the Borch reduction are mild, sodium cyanoborohydride is highly toxic, as are its byproducts. The pH was maintained by addition of HCl and/or KOH as needed using bromocresol green as an indicator.
위험도
Flammable solid. Dust may form explosive mixture with air. Fire-water run-off in sewers may create fire or explosion hazard. This product is toxic. Severe corrosive hazard. Water used for fire extinguishing, which has been in contact with the product, may be corrosive.Purification Methods
Sodium cyanoborohydride (10g) is dissolved in THF (80mL) and 1 M methanolic hydrochloric acid is added until pH reaches 9. The solution is then poured with stirring into dioxan (250mL). The precipitate is collected and stirred for 2 h in ethyl acetate (250mL). This solution is filtered, heated to reflux on a steam bath and then dioxan (150mL) is added slowly with swirling. This solution is slowly cooled to room temperature, chilled and filtered. The crystalline dioxan complex is dried in vacuo for 4 h at room temperature, then for 4 h at 80° C; yield 6.74g, purity >98% sodium cyanoborohydride by iodometric titration.나트륨 사이아노브로민하이드라이드(나트륨 시아노브롬하이드리드) 준비 용품 및 원자재
원자재
준비 용품
N,N-Dimethylpiperidin-4-amine
N-isopropyl-2-(piperazin-1-yl)pyridin-3-amine
1-CYCLOPROPYLPIPERAZINE
3-Dimethylaminophenylboronic acid
Enalapril
NETILMICIN
7-BROMO-1,2,3,4-TETRAHYDRO-QUINOLINE HYDROCHLORIDE
6-bromo-3,4-dihydro-3-nitro-2H-chromene
Lisinopril Dihydrate
4-N-Boc-4-N-Methyl-aminopiperidine
1-(CYCLOPROPYLMETHYL)PIPERAZINE
2-AMINOCYCLOHEXANECARBONITRILE
1-Piperonylpiperazine
N-METHYL-P-ANISIDINE
ethyl indoline-6-carboxylate
tert-Butyl 4-hydrazinylpiperidine-1-carboxylate
4-(1-Pyrrolidinyl)piperidine
INDOLINE-6-CARBOXYLIC ACID
N-Boc-4-(Cyclopropylmethyl)piperazine
1-Boc-4-Methylaminopiperidine
4-Aminopyrimidine
1-(3-PYRROLIDINOPROPYL)PIPERAZINE
나트륨 사이아노브로민하이드라이드(나트륨 시아노브롬하이드리드) 공급 업체
글로벌( 597)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Guanlang Biotechnology Co,.LTD | +8619930503252 |
daisy@crovellbio.com | China | 5961 | 58 |
| BTC pharm India | +918790379245 |
banny.janga@btcpharm.com | India | 1269 | 58 |
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 |
admin@firsky-cn.com | China | 436 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 |
market18@leapchem.com | China | 24738 | 58 |
| Nanjing Deda New Material Technology Co., Ltd | +8613223293093 |
bella@njdeda.com | China | 82 | 58 |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 |
sales@myuchem.com | China | 6708 | 58 |
| Suzhou Sanyi Polymer Chemical Technology Co., Ltd. | +8615571922873 |
15571922873@163.com | China | 106 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 |
deasea125996@gmail.com | China | 2503 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 994 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 18223 | 58 |