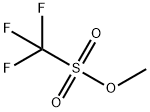
Methyl trifluoromethanesulfonate
|
|
Methyl trifluoromethanesulfonate 속성
- 끓는 점
- 94-99 °C(lit.)
- 밀도
- 1.45 g/mL at 25 °C(lit.)
- 굴절률
- n
20/D 1.326(lit.)
- 인화점
- 101 °F
- 저장 조건
- 2-8°C
- 용해도
- 클로로포름(적게), 에틸아세테이트(약간)에 용해됨
- 물리적 상태
- 액체
- 색상
- 무색~황색으로 투명함
- Specific Gravity
- 1.450
- 감도
- Air Sensitive
- BRN
- 774772
- 안정성
- 흡습성, 수분 민감성, 휘발성
- InChI
- InChI=1S/C2H3F3O3S/c1-8-9(6,7)2(3,4)5/h1H3
- InChIKey
- OIRDBPQYVWXNSJ-UHFFFAOYSA-N
- SMILES
- C(F)(F)(F)S(OC)(=O)=O
- CAS 데이터베이스
- 333-27-7(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | C,T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 10-34-20/21/22 | ||
| 안전지침서 | 26-36/37/39-45-16 | ||
| 유엔번호(UN No.) | UN 2920 8/PG 2 | ||
| WGK 독일 | 3 | ||
| F 고인화성물질 | 10 | ||
| 위험 참고 사항 | Highly Toxic/Corrosive | ||
| TSCA | T | ||
| 위험 등급 | 3.2 | ||
| 포장분류 | III | ||
| HS 번호 | 29049020 |
Methyl trifluoromethanesulfonate C화학적 특성, 용도, 생산
화학적 성질
clear colourless to yellow liquid용도
Methyl trifluoromethanesulfonate is used as a powerful methylating reagent in chemistry. Further, it is used in the conversion of amines to methyl ammonium triflates. In addition, it is also used in Suzuki reaction.주요 응용
Methyl trifluoromethanesulfonate, a methylating agent, is commonly used for the pre-methylation of polysaccharides under mild basic conditions.In the determination of polysulfides, zerovalent sulfur in sulfide-rich water wells, and polysulfide species in electrolyte of a lithium–sulfur battery using chromatography-based techniques.
In reactions with potassium enolates.
For the conversion of amines to methyl ammonium triflates.
Additionally, methyl trifluoromethanesulfonate has been utilized in the production of 2-Benzyloxy-1-methylpyridinium trifluoromethanesulfonate.
일반 설명
Methyl trifluoromethane sulfonate is a brown liquid. Insoluble in water. (NTP, 1992) This material is a very reactive methylating agent, also known as methyl triflate.공기와 물의 반응
Highly flammable. Insoluble in water.반응 프로필
Methyl trifluoromethanesulfonate is sensitive to heat. . Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.건강위험
ACUTE/CHRONIC HAZARDS: When heated to decomposition Methyl trifluoromethanesulfonate emits toxic fumes.화재위험
Methyl trifluoromethanesulfonate is combustible.Toxicology
Methyl trifluoromethanesulfonate is extremely hazardous. All possible precautions should be taken to avoid inhalation or absorption through the skin. This material is extremely destructive to the tissue of the mucous membranes and upper respiratory tract, eyes, and skin. Inhalation may be fatal as a result of spasm, inflammation, and edema of the larynx and bronchi, chemical pneumonitis, and pulmonary edema. Use in a fume hood. Although there has yet to be a reported human fatality, several cases were reported for methyl fluorosulfonate (LC50 (rat, 1 h) = 5 ppm), and methyl triflate is expected to have similar toxicity based on available evidence.Synthesis
Methyl trifluoromethanesulfonate is commercially available, however it may also be prepared in the laboratory by treating dimethyl sulfate with triflic acid.CF3SO2OH + (CH3O)2SO2 → CF3SO2OCH3 + CH3OSO2OH
Purification Methods
Methyl trifluoromethanesulfonate (methyl triflate) [333-27-7] M 164.1, b 97 -97.5o/736mm, 9 9o/~760mm, 100-102o/~760mm, d 4 1.496, n D 1.3238. It is a strong methylating agent but is corrosive and POISONOUS. Fractionate it carefully and collecting the middle fraction (use an efficient fume cupboard) and keep away from moisture. It is a POWERFUL ALKYLATING AGENT and a strong IRRITANT. [IR: Gramstad & Haszeldine J Chem Soc 173 1956 , J Chem Soc 4069 1957 .] Trifluoromethanesulfonic acid (triflic acid) [1493-13-6] M 151.1, boils higher (b 162o/atm), has a pKa of 3.10, and is TOXIC and hygroscopic. [Hansen J Org Chem 30 4322 1965, Kurz & El-Nasr J Am Chem Soc 104 5823 1982, Beilstein 3 IV 34.]Methyl trifluoromethanesulfonate 준비 용품 및 원자재
원자재
준비 용품
Methyl trifluoromethanesulfonate 공급 업체
글로벌( 392)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Wuhan Huahan Dingcheng New Material Technology Co., Ltd | +undefined-027-81388558 +undefined086-1878077615 |
huahandingcheng@sina.com | China | 342 | 58 |
| Allfluoro pharmaceutical co. ltd. | +86-400-0216110 +86-15958047586 |
sales@allfluoro.com | China | 11385 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +86-15531157085 +86-15531157085 |
abby@chuanghaibio.com | China | 8808 | 58 |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12816 | 58 |
| airuikechemical co., ltd. | +86-18353166132 +86-18353166132 |
sales02@airuikechemical.com | China | 983 | 58 |
| Hebei Zhuanglai Chemical Trading Co Ltd | +86-16264648883 +86-16264648883 |
niki@zlchemi.com | China | 3720 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-+86-371-66670886 |
info@dakenam.com | China | 19801 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21630 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 |
jack.li@time-chemicals.com | China | 1803 | 55 |
| Hangzhou FandaChem Co.,Ltd. | 008657128800458; +8615858145714 |
FandaChem@Gmail.com | China | 4420 | 55 |