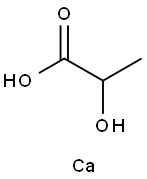
유산칼슘
|
|
유산칼슘 속성
- 용해도
- 물에 용해되고 끓는 물에 잘 용해되며 에탄올에는 매우 약간 용해됩니다(96%).
- 수소이온지수(pH)
- 7.56(1 mM solution);7.97(10 mM solution);8.28(100 mM solution);8.5(1000 mM solution)
- 냄새
- 냄새 없는
- InChI
- InChI=1S/C3H6O3.Ca.2H/c1-2(4)3(5)6;;;/h2,4H,1H3,(H,5,6);;;
- InChIKey
- KSDPLTNUUQNXLV-UHFFFAOYSA-N
- SMILES
- C(O)(C)C(=O)O.[Ca]
- LogP
- -0.698 (est)
- CAS 데이터베이스
- 814-80-2(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험 카페고리 넘버 | 36/37/38 | ||
|---|---|---|---|
| 안전지침서 | 26-36/37/39 | ||
| TSCA | Yes | ||
| 유해 물질 데이터 | 814-80-2(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-20846 |
| 그림문자(GHS): |

|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Warning | ||||||||||||||||||||||||||||
| 유해·위험 문구: |
|
||||||||||||||||||||||||||||
| 예방조치문구: |
|
유산칼슘 C화학적 특성, 용도, 생산
물성
백색의 분말 내지는 결정으로, 냄새가 없거나 미약한 특이한 냄새가 있다.120°C에서 무수 물이 된다. 각종 칼슘염 중에서는 용해도가 높다.용도
영양보강제 (칼슘제)로서 각종식품(과자류, 청량음료, 된장, 두부, 분유, 빵 등) 및 의약 품 등에 첨가된다. 조직 보강제로서 각종 농산 가공 통조림, 단무지 절임 등에 사용된다.개요
Calcium lactate is a black or white crystalline salt made by the action of lactic acid on calcium carbonate. It is used in foods (as an ingredient in baking powder) and given medicinally. Its E number is E327. It is created by the reaction of lactic acid with calcium carbonate or calcium hydroxide.Calcium lactate is often found in aged cheeses. Small crystals of it precipitate out when lactic acid is converted into a less soluble form by the bacteria active during the ripening process.
In medicine, calcium lactate is most commonly used as an antacid and also to treat calcium deficiencies. Calcium lactate can be absorbed at various pHs and does not need to be taken with food for absorption for these reasons.
Calcium lactate is added to sugar-free foods to prevent tooth decay. When added to chewing gum containing xylitol, it increases the remineralization of tooth enamel.It is also added to fresh-cut fruits such as cantaloupes to keep them firm and extend their shelf life, without the bitter taste caused by calcium chloride, which can also be used for this purpose.
화학적 성질
Calcium lactate occurs as white or almost white, crystalline or granular powder. It is slightly efflorescent.용도
Calcium Lactate is the calcium salt of lactic acid which is soluble in water. it has a solubility of 3.4 g/100 g of water at 20°c and is very soluble in hot water. it is available as a monohydrate, trihydrate, and pentahydrate. the trihydrate and pentahydrate have solubili- ties of 9 g in 100 ml of water at 25°c. it contains approximately 14% calcium. it is used to stabilize and improve the texture of canned fruits and vegetables by converting the labile pectin to the less solu- ble calcium pectate. it thereby prevents structural collapse during cooking. it is used in angel food cake, whipped toppings, and meringues to increase protein extensibility which results in an increase of foam volume. it is also used in calcium fortified foods such as infant foods and is used to improve the properties of dry milk powder.생산 방법
Calcium lactate is prepared commercially by neutralization with calcium carbonate or calcium hydroxide of lactic acid obtained from fermentation of dextrose, molasses, starch, sugar, or whey.위험도
A poison.Pharmaceutical Applications
Calcium lactate is used as a bioavailability enhancer and nutrient supplement in pharmaceutical formulations.A spray-dried grade of calcium lactate pentahydrate has been used as a tablet diluent in direct compression systems, and has been shown to have good compactability. The properties of the pentahydrate form have been considered superior to those of calcium lactate trihydrate when used in direct compression tablet formulations. Tablet properties may be affected by the hydration state of the calcium lactate and particle size of the material: reducing particle size increased crushing strength, whereas storage of tablets at elevated temperature resulted in dehydration accompanied by a reduction in crushing strength.
Calcium lactate has also been used as the source of calcium ions in the preparation of calcium alginate microspheres for controlled- release delivery of active agents. It has been shown to result in lower calcium concentrations in the finished microspheres when compared with calcium acetate.
Therapeutically, calcium lactate has been used in preparations for the treatment of calcium deficiency.
Safety
Calcium lactate was found to have no toxic or carcinogenic effects when dosed at levels of 0%, 2.5%, and 5% in drinking water to male and female rats for 2 years.저장
Calcium lactate can exist in a number of hydration states, which are characterized as anhydrous, monohydrate, trihydrate, and pentahydrate. Dehydration of the pentahydrate form is rapid at temperatures of 558℃ and above. Dehydration is reported to be accompanied by some loss of crystallinity. Tablet crushing strength was reported to be reduced following dehydration of calcium lactate pentahydrate.Purification Methods
Crystallise it from warm water (10mL/g) by cooling to 0o. [Beilstein 3 IV 636.]비 호환성
Calcium salts, including the lactate, can display physical incompatibility with phosphate in the diet or therapeutic preparations, for example in enteral feed mixtures.Regulatory Status
GRAS listed except for infant foods/formulas. Accepted as a food additive in Europe. Calcium lactate (anhydrous) is included in the FDA Inactive Ingredients Database (vaginal, tablet). It is used in oral dosage forms. Included in vaginal pessary formulations licensed in the UK.유산칼슘 준비 용품 및 원자재
원자재
준비 용품
유산칼슘 공급 업체
글로벌( 449)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hebei Chuanghai Biotechnology Co., Ltd | +8617732866630 |
abby@chuanghaibio.com | China | 8773 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 +86-13131129325 |
sales1@chuanghaibio.com | China | 5251 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 |
mina@chuanghaibio.com | China | 18126 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 |
lisa@kingfinertech.com | China | 3003 | 58 |
| Anhui Ruihan Technology Co., Ltd | +8617756083858 |
daisy@anhuiruihan.com | China | 973 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20207 | 58 |
| Shaanxi TNJONE Pharmaceutical Co., Ltd | +86-17396673057 |
linda@tnjone.com | China | 1143 | 58 |
| Hebei Zhuanglai Chemical Trading Co.,Ltd | +8613343047651 |
admin@zlchemi.com | China | 3692 | 58 |
| Lianyungang Kaiyu Environmental Tech Co., Ltd. | 18052309100 +8618052309100 |
carlos@khonorchem.com | China | 23 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21622 | 55 |