|
|
| | 2-Amino-6-chloropurine Riboside Basic information |
| | 2-Amino-6-chloropurine Riboside Chemical Properties |
| Melting point | 165-167 °C (dec.)(lit.) | | Boiling point | 729.9±70.0 °C(Predicted) | | density | 1.8359 (rough estimate) | | refractive index | -38 ° (C=0.1, H2O) | | storage temp. | Keep in dark place,Inert atmosphere,Store in freezer, under -20°C | | solubility | DMSO, Methanol | | pka | 13.05±0.70(Predicted) | | form | Powder | | color | White to Off-white | | InChIKey | TXWHPSZYRUHEGT-ACJOCUEISA-N | | CAS DataBase Reference | 2004-07-1(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 24/25-36-26 | | WGK Germany | 3 | | Hazard Note | Irritant | | HS Code | 29349990 |
| | 2-Amino-6-chloropurine Riboside Usage And Synthesis |
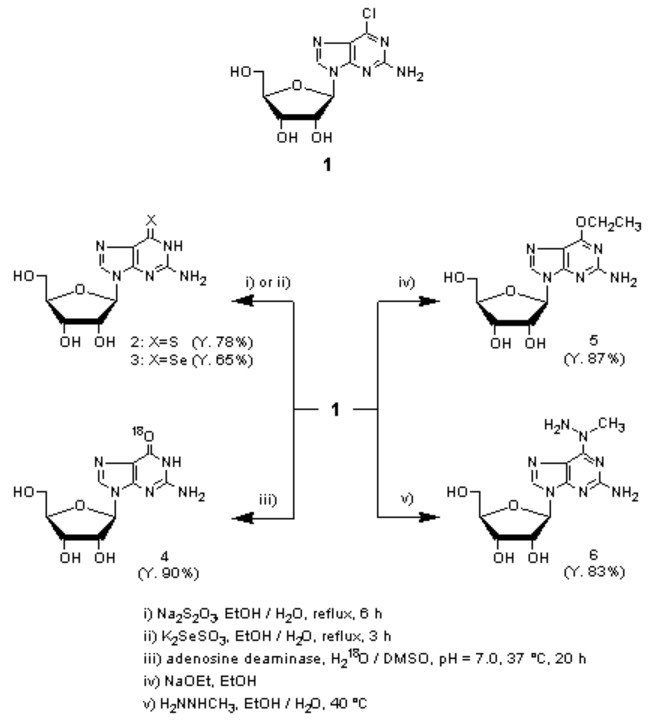
| Chemical Properties | Colourless Crystalline Solid | | Uses | 2-Amino-6-chloropurine riboside (1) is a a novel class of inhibitors of endogenous protein degradation for the preparation of a number of important purine nucleoside derivatives. For example, 6-thioguanosine 2 and 6-selenoguanosine 3 were prepared from a reaction of 1 and the corresponding sodium thiosulfate or potassium selenosulfate.1) 18O-labeled guanosine 4 was incubated from 1 with adenosine deaminase in (18O)-water.2) 2-Amino-6-ethoxypurine riboside 5 and 2-amino-N6-amino-N6-methyladenosine 6 were prepared by treatment of 1 with nucleophiles, sodium ethoxide and methylhydrazine, respectively.3,4) 2-Amino-N6-substituted purine analogues, as typified by 6, were reported as anti-malarial active compounds.
 | | References | [1] A. JANKOWSKI L. T D Wise. Sodium Thiosulfate and Potassium Selenosulfate as Reagents to Prepare Thio-and Selenopurine Nucleosides[J]. Nucleosides, Nucleotides & Nucleic Acids, 1989, 8 1: 339-348. DOI:10.1080/07328318908054179.
[2] SUNDEEP RAYAT. 5-Cyanoimino-4-oxomethylene-4,5-dihydroimidazole and 5-Cyanoamino-4-imidazolecarboxylic Acid Intermediates in Nitrosative Guanosine Deamination: Evidence from 18O-Labeling Experiments[J]. Journal of the American Chemical Society, 2004, 126 32: 9960-9969. DOI:10.1021/ja049835q.
[3] MORRIS J. ROBINS. Nucleic acid related compounds. 114. Synthesis of 2,6-(disubstituted)purine 2′,3′-dideoxynucleosides and selected cytotoxic, anti-hepatitis b, and adenosine deaminase substrate activities[J]. Journal of Heterocyclic Chemistry, 2009, 38 6: 1297-1306. DOI:10.1002/jhet.5570380609.
[4] KATHLEEN TOO . Anti-malarial activity of N6-modified purine analogues[J]. Bioorganic & Medicinal Chemistry, 2007, 15 16: Pages 5551-5562. DOI:10.1016/j.bmc.2007.05.038.
[5] ALICJA STACHELSKA-WIERZCHOWSKA. Tri-Cyclic Nucleobase Analogs and their Ribosides as Substrates of Purine-Nucleoside Phosphorylases. II Guanine and Isoguanine Derivatives.[J]. Molecules, 2019, 24 8. DOI:10.3390/molecules24081493.
[6] SHIH-HSI CHU Ming Y C Chyng Yann Shiue. Synthesis and cytotoxicity of 6-selenopurine arabinoside and related compounds[J]. Journal of pharmaceutical sciences, 1975, 64 8: 1343-1346. DOI:10.1002/jps.2600640818. |
| | 2-Amino-6-chloropurine Riboside Preparation Products And Raw materials |
|