|
|
| | 3-methyl-1H-indazol-6-amine Basic information |
| Product Name: | 3-methyl-1H-indazol-6-amine | | Synonyms: | 3-methyl-1H-indazol-6-amine;1H-Indazol-6-aMine,3-Methyl-;3-Methyl-2H-indazol-6-aMine;CC1=NNC2=C1C=CC(N)=C2;6-AMino-3-Methyl (1H)indazole;6-AMINO-3-MRTHYL(1H)INDAZOLE;3-Methyl-1H-indazol-6-ylamine ,97%;3-Methyl-1H-indazol-6-ylamine | | CAS: | 79173-62-9 | | MF: | C8H9N3 | | MW: | 147.18 | | EINECS: | | | Product Categories: | | | Mol File: | 79173-62-9.mol | 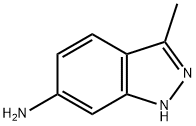 |
| | 3-methyl-1H-indazol-6-amine Chemical Properties |
| Boiling point | 376.4±22.0 °C(Predicted) | | density | 1.295±0.06 g/cm3(Predicted) | | storage temp. | Keep in dark place,Inert atmosphere,Room temperature | | pka | 16.00±0.40(Predicted) |
| | 3-methyl-1H-indazol-6-amine Usage And Synthesis |
| Description | 3-methyl-1H-indazol-6-amine is an organic compound that belongs to the group of diazo compounds. It is a benzene derivative with three alkoxy groups, which are substituted with an alkyl or an aryl group. 3-Methyl-1H-indazol-6-amine can be synthesized by coupling the corresponding chlorides with toluene and diazonium salts in an alkaline solution. The fibre used for this synthesis has been shown to have aryl and alkyl groups. This compound reacts with acid as hydrogen chloride, resulting in the release of carbon dioxide gas. 3-Methyl-1H-indazol-6-amine also reacts readily with water and alcohols, forming indazole hydrochloride and benzoic acid respectively. | | Chemical Properties | Light yellow to brown solid |
| | 3-methyl-1H-indazol-6-amine Preparation Products And Raw materials |
|