|
|
| | 2-amino-5-methylbenzenesulfonamide Basic information |
| Product Name: | 2-amino-5-methylbenzenesulfonamide | | Synonyms: | 2-amino-5-methylbenzenesulfonamide;Benzenesulfonamide, 2-amino-5-methyl- (9CI);NSC57677;Benzenesulfonamide, 2-amino-5-methyl-;2-azanyl-5-methyl-benzenesulfonamide;2-amino-4-methylbenzenesulfonamide;2-amino-5-methylbenzenesulfomide;Pazopanib Impurity 60 | | CAS: | 609-55-2 | | MF: | C7H10N2O2S | | MW: | 186.23 | | EINECS: | | | Product Categories: | SULFONAMIDE | | Mol File: | 609-55-2.mol | 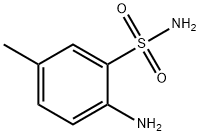 |
| | 2-amino-5-methylbenzenesulfonamide Chemical Properties |
| storage temp. | 2-8°C | | InChI | InChI=1S/C7H10N2O2S/c1-5-2-3-6(8)7(4-5)12(9,10)11/h2-4H,8H2,1H3,(H2,9,10,11) | | InChIKey | GKTVIFGJASUBGA-UHFFFAOYSA-N | | SMILES | C1(S(N)(=O)=O)=CC(C)=CC=C1N |
| | 2-amino-5-methylbenzenesulfonamide Usage And Synthesis |
| Uses | 2-amino-5-methylbenzenesulfonamide is an important organic reagent that can be used as a building block for the synthesis of many organic compounds. It could reacted with triethyl orthoformate to give 7-Methyl-4H-1,2,4-benzothiadiazine 1,1-dioxide by ring closure. It was an intermediate of potent AMPA receptor potentiators[1].
| | Synthesis | A solution of 7-methyl-3-oxo-3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxide (10, 10 g, 47,12 mmol) in sulfuric acid 50% (150 mL) was refluxed until complete dissolution of the suspension. At the end of the reaction, the solution was adjusted to pH 7 with an aqueous solution of concentrated NaOH. The solvent was removed by distillation under reduced pressure. The residue was triturated with methanol and the salts were eliminated by filtration. The filtrate was concentrated to dryness under reduced pressure and the residue was triturated in water (a minimum). The resulting precipitate was collected by filtration, washed with water, and dried to afford 2-amino-5-methylbenzenesulfonamide[1]. | | References | [1] Dintilhac G, et al. New substituted aryl esters and aryl amides of 3,4-dihydro-2H-1,2,4-benzothiadiazine 1,1-dioxides as positive allosteric modulators of AMPA receptors. RSC Medicinal Chemistry, 2011; 2: 509-523. |
| | 2-amino-5-methylbenzenesulfonamide Preparation Products And Raw materials |
|