|
|
| | GDC-0623 Basic information |
| Product Name: | GDC-0623 | | Synonyms: | RG 7421;5-(2-Fluoro-4-iodophenylamino)imidazo[1,5-a]pyridine-6-carboxylic acid N-(2-hydroxyethoxy)amide;GDC-0623 (5-((2-Fluoro-4-Iodophenyl)amino)-N-(2-Hydroxyethoxy)imidazo[1,5-a]pyridine-6-Carboxamide);5-(2-Fluoro-4-iodophenylamino)imidazo[1,5-a]pyridine-6-carboxylic acid N-(2-hydroxyethoxy)amide RG 7421 GDC-0623;GDC-0623;MEK inhibitor 1;G-868;5-((2-FLUORO-4-IODOPHENYL)AMINO)-N-(2-HYDROXYETHOXY)IMIDAZO[1,5-A]PYRIDINE-6-CARBOXAMIDE | | CAS: | 1168091-68-6 | | MF: | C16H14FIN4O3 | | MW: | 456.21 | | EINECS: | 604-604-1 | | Product Categories: | | | Mol File: | 1168091-68-6.mol | 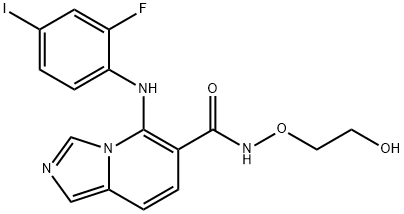 |
| | GDC-0623 Chemical Properties |
| density | 1.81±0.1 g/cm3(Predicted) | | storage temp. | Store at -20°C | | solubility | insoluble in H2O; ≥16.85 mg/mL in DMSO; ≥2.69 mg/mL in EtOH with ultrasonic | | form | solid | | pka | 14.17±0.10(Predicted) | | color | Light yellow to gray |
| | GDC-0623 Usage And Synthesis |
| Description | GDC-0623 is a potent, ATP-uncompetitive inhibitor of MEK1 (Ki = 0.13 nM in the presence of ATP). It inhibits the proliferation of A375 BRAF(V600E) and HCT116 KRAS(G13D)-mutant cancer cell lines with EC50 values of 7 and 42 nM, respectively. GDC-0623 inhibits ERK and BIM phosphorylation resulting in upregulation and stabilization of pro-apoptotic BIM protein in KRAS mutant and wild-type HCT116 cells and in KRAS mutant GW620 cells. | | Definition | ChEBI: GDC-0623 is a member of the class of imidazopyridines that is imidazo[1,5-a]pyridine substituted by (2-fluoro-4-iodophenyl)amino and (2-hydroxyethoxy)aminoacyl groups at positions 5 and 6. It is a potent ATP non-competitive inhibitor of MEK1 (Ki = 0.13nM) and also has efficacy against both mutant BRAF and mutant KRAS. It is in clinical development for treatment of patients with locally advanced or metastatic solid tumors. It has a role as an EC 2.7.12.2 (mitogen-activated protein kinase kinase) inhibitor, an antineoplastic agent and an apoptosis inducer. It is a substituted aniline, a member of monofluorobenzenes, an organoiodine compound, an imidazopyridine, a secondary amino compound, a hydroxamic acid ester and a primary alcohol. | | target | MEK1 | | references | [1] hatzivassiliou g, haling j r, chen h, et al. mechanism of mek inhibition determines efficacy in mutant kras-versus braf-driven cancers. nature, 2013, 501(7466): 232-236. |
| | GDC-0623 Preparation Products And Raw materials |
|