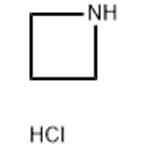
- Azetidine hydrochloride
-

- $0.00 / 25kg
-
2025-03-03
- CAS:36520-39-5
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 10000KGS
- Azetidine hydrochloride
-
- $0.00 / 1kg
-
2023-11-01
- CAS:36520-39-5
- Min. Order: 0.10000000149011612kg
- Purity: 99.0%min
- Supply Ability: 200kg
- Azetidine hydrochloride
-

- $15.00 / 1KG
-
2021-07-13
- CAS:36520-39-5
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
|
| | Azetidine hydrochloride Basic information |
| | Azetidine hydrochloride Chemical Properties |
| Melting point | >300 °C(lit.) | | Fp | 152 °C | | storage temp. | Inert atmosphere,2-8°C | | form | powder to crystal | | color | White to Almost white | | Water Solubility | Soluble in water (103.8°C at 760 mmHg). | | Sensitive | Hygroscopic | | InChI | InChI=1S/C3H7N.ClH/c1-2-4-3-1;/h4H,1-3H2;1H | | InChIKey | HGQULGDOROIPJN-UHFFFAOYSA-N | | SMILES | C1CNC1.Cl | | CAS DataBase Reference | 36520-39-5(CAS DataBase Reference) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 26 | | RIDADR | UN1325 | | WGK Germany | 3 | | HazardClass | IRRITANT, HYGROSCOPIC | | HS Code | 29339900 |
| | Azetidine hydrochloride Usage And Synthesis |
| Chemical Properties | White powder | | Uses | Basic building block for synthesizing azetidines.1,2 | | Uses | Azetidine hydrochloride is a building block for azetidine synthesis and a pharmaceutical intermediate. | | Uses | Azetidine hydrochloride is a four-membered ring nitrogen-containing heterocycle. Useful building block in the synthesis of polypeptides and other nitrogen containing compounds with potential biological properties. | | General Description | Azetidine derivatives are an essential class of aza-heterocyclic compounds with various biological activities and have been widely used as building blocks in pharmacological research. Although azetidine has the simplest structure, it is only commercially available in small quantities and at a high price. In addition, this compound is hard to synthesize for several reasons, such as cyclization of the four-membered ring, stability of the ring, and low boiling point[1]. | | References | [1] Jian Sun, Qi Sun, S. Gong. “A Novel Strategy for the Synthesis of Azetidine.” Advanced Materials Research 6 1 (2013): 135–138.
|
| | Azetidine hydrochloride Preparation Products And Raw materials |
|