Gallium: Crystal Structure, Mineral materials, Chemical reactions and Major uses
May 28,2024
Gallium has a silvery blue appearance. It has a low melting point at 29.7646℃ and forms a liquid state that is denser than the solid. Gallium expands by around 3% when it solidifies and is normally not stored in glass or metal containers. This is to prevent rupture of the container when gallium changes state from liquid to solid. Gallium also diffuses into grain boundaries of materials like aluminum-zinc alloys and steel making them brittle. Gallium is a member of Group 13 or Boron family with an atom electronic configuration of [Ar]3d104s2 4p1 . Its compounds are found primarily in the 13 oxidation state, although the 11 oxidation state is also known.
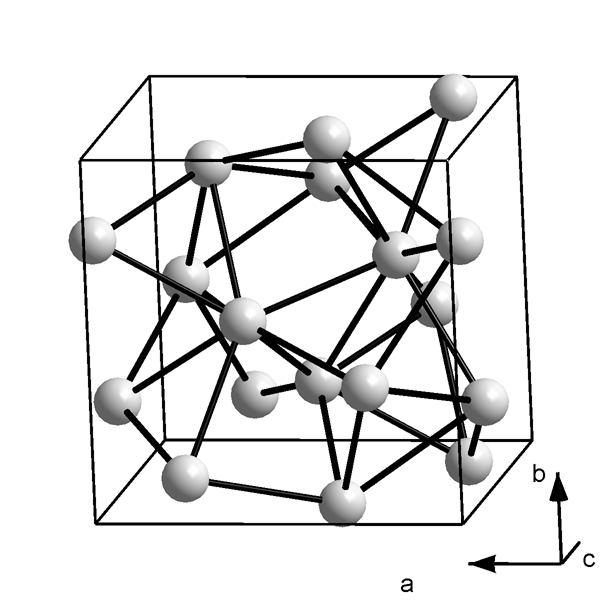
Crystal Structure
Gallium crystallizes in a unique orthorhombic (pseudotetragonal) structure with 8 atoms in the unit cell. Each gallium
atom in the unit cell has one nearest neighbor at 244 pm while the other six atoms are farther away. The two nearest
neighbors are covalently bonded. Hence, gallium clusters are considered as having Ga2 dimers as their building block.
This structure forms because of interactions between the single 4p electrons of gallium atoms which is farther away
from the nucleus than the 4s electrons and the [Ar]3d10 core. Gallium is fairly reactive, combining with most nonmetals
at high temperatures. Interests in its chemistry are not only due to the important structure and properties of its compounds but also due to academic curiosity.
Mineral materials
Ga does not occur as a free element in the Earth’s crust, and the few high-concentration minerals, such as gallite
(CuGaS2), are too rare to be mined as a primary source. In total only seven minerals are known to contain gallium. Three sulfides exist with Ga, gallite (CuGaS2), ishiharaite
((Cu,Ga,Fe,In,Zn)S), and zincobriartite (Cu2(Zn,Fe)(Ge,Ga)S4). Two oxide class minerals contain Ga, so¨hngeite
(Ga(OH)3) (Fig. 2) and tsumgallite (GaOOH). The phosphate class is represented by two minerals, gallobeudantite (PbGa3(AsO4)(SO4)(OH)6) and galloplumbogummite (Pb(Ga,Al,Ge)3(PO4)2(OH)6).

Chemical reactions
The metal gallium is stable in dry air, oxidizing slowly until it forms an oxide film that prevents it from further oxidation. It burns in air or oxygen to form the white oxide, Ga2O3. This oxide exists in many polymorphs and can be
reduced to the metal when heated at high temperatures in hydrogen. It gives the lower oxide Ga2O when heated with
gallium metal at 700℃.
Gallium in its vapor form reacts with hydrogen under appropriate conditions to give metal hydrides with the formula MH or MH3. These hydrides, however, are transient and convert back to its elements. GaH3 cannot be prepared or isolated easily but Ga2H6 or H2Ga(H)2GaH2 have been prepared and characterized to some extent. Like aluminum, gallium(III) hydride, GaH3, known as gallane, may also be produced by reacting lithium gallanate (LiGaH4) with gallium(III) chloride at 230℃.
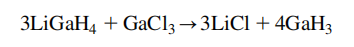
In the presence of dimethyl ether as solvent, GaH3 polymerizes to (GaH3)n. If no solvent is used, the dimer Ga2H6
(digallane) is formed as a gas. Its structure is similar to diborane, having two hydrogen atoms bridging the two gallium
centers, unlike α-AlH3 in which aluminum has a coordination number of 6. Gallane is unstable above 210℃, decomposing to elemental gallium and hydrogen. Gallium forms compounds with nitrogen but not through direct combination
of the elements. GaN can be grown when gallium/sodium melt are subjected to 100 atmospheres of N2 at 750℃. GaN
thin films can be prepared by molecular-beam epitaxy on silicon carbide (SiC) or sapphire substrates. GaN crystals can
also be formed by injecting ammonia gas into molten gallium at 900℃~ 980℃ at normal atmospheric pressures or by
reacting Ga2O3 with ammonia at elevated temperatures of the order of 1000℃.
Ga2O is a very strong reducing agent, which can reduce H2SO4 to H2S. It disproportionates at 800℃ back to gallium
and Ga2O3. Gallium(III) sulfide, Ga2S3, has 3 possible crystal modifications. It can be formed by the reaction of gallium
with hydrogen sulfide (H2S) at 950℃. Instead, Ga(OH)3 can be used at 747℃.
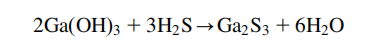
Reacting a mixture of alkali metal carbonates and Ga2O3 with H2S results in the formation of thiogallates with the [Ga2S4] 22 anion. Strong acids decompose these salts, releasing H2S during the reaction. The mercury salt, HgGa2S4, can be utilized as a phosphor. Additionally, gallium can form sulfides in lower oxidation states, such as gallium(II) sulfide and the green gallium(I) sulfide, the latter of which is produced from the former by heating to 1000℃ under a stream of nitrogen. The other binary chalcogenides, Ga2Se3 and Ga2Te3, have the zincblende structure (sphalerite, ZnS). They are all semiconductors but are easily hydrolyzed and have limited value.
Major uses
Extremely high-purity ( . 99.9999%) Ga is commercially available for the semiconductor industry. Gallium arsenide
(GaAs) and gallium nitride (GaN) used in electronic components represented about 98% of the Ga consumption in the
United States. Around 66% of semiconductor Ga is used in integrated circuits (mostly GaAs), for example, the manufacture of ultra-high-speed logic chips and MESFETs (metal semiconductor field-effect transistor) for low-noise
microwave preamplifiers in cell phones. Around 20% of this Ga is used in optoelectronics. GaAs and GaN can be found in various optoelectronic devices. Aluminum gallium arsenide (AlGaAs) is utilized in high-power infrared laser diodes.
The semiconductors GaN and indium gallium nitride
(InGaN) are used in blue and violet optoelectronic devices, mostly laser diodes and LEDs, for example, GaN 405 nm
diode lasers are the violet light source for higher-density Blu-ray Disc compact data disc drives.
Ga easily alloys with most metals and is used for the production of low-melting alloys. The virtually eutectic alloy of Ga, In, and Sn is a room temperature liquid used in medical thermometers. Ga alloys have been assessed as substitutes for Hg dental amalgams, but these materials have not yet been widely accepted. Since Ga wets glass or porcelain, it can be used to produce brilliant mirrors. When the wetting action of Ga alloys is not needed (as in Galinstan glass thermometers), the glass must be covered with a transparent layer of Ga(III) oxide. The Pu used in nuclear weapon pits is stabilized in the δ phase and made machinable by alloying with Ga.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringAs a metal, titanium is known for its high strength-to-weight ratio. It is a strong metal with low density that is rather ductile (in particular in the absence of oxygen), lustrous, and metallic-white in color.....
May 28,2024Inorganic chemistryGallium
7440-55-3You may like
- Sliver Gallium
-

- $360.00/ kg
- 2025-02-28
- CAS:7440-55-3
- Min. Order: 5kg
- Purity: 98%
- Supply Ability: 20T
- Gallium
-

- $199.00 / 1KG
- 2024-11-07
- CAS:7440-55-3
- Min. Order: 100KG
- Purity: 99.9999%
- Supply Ability: 10 ton /month
- Metal Gallium
-

- $400.00 / 10kg
- 2024-09-19
- CAS:7440-55-3
- Min. Order: 10kg
- Purity: 99.99%
- Supply Ability: 200tons/Month






