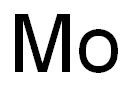The History of the Discovery of Molybdenum
May 28,2024
Molybdenite—the principal ore from which molybdenum is now separated—was formerly known as molybdena.

Molybdena was confused with and often used as if it were graphite (a form of carbon, C). Similar to graphite, molybdenite can be applied to blacken a surface or as a solid lubricant. Even when molybdena was distinguishable from graphite, it was still confused with the common lead ore now known as galena (PbS); the name comes from Ancient Greek Moλυβδoς molybdos, meaning lead. (The Greek word itself has been suggested as a loanword from Anatolian Luvian and Lydian languages.)
In the West in 1754, Swedish chemist and mineralogist Bengt Andersson Qvist (October 21, 1726 to October 14, 1799) analyzed a sample of molybdenite and showed that it did not contain lead and therefore was not galena (Quist, 1754). By 1778, Swedish Pomeranian and German pharmaceutical chemist Carl Wilhelm Scheele (December 9, 1742 to May 21, 1786) stated firmly that molybdena was neither galena nor graphite. In its place, Scheele correctly put forward that molybdena was an ore of a separate new element, which he called molybdenum for the mineral in which it was found, and from which it might be separated (Scheele, 1779).
Swedish chemist Peter Jacob Hjelm (October 2, 1746 to October7, 1813) successfully separated molybdenum using carbon and linseed oil in 1781 (Hjelm, 1789). For the next 100 years, molybdenum had no commercial use. It was rather uncommon, the pure metal was difficult to isolate, and the required techniques of metallurgy were not developed yet. Early molybdenum steel alloys exhibited great potential of increased hardness but attempts to produce the alloys on a large scale were hindered by variable results, a tendency toward brittleness, and recrystallization.
In 1906, American physicist and engineer William D. Coolidge (October 23, 1873 to February 3, 1975) filed a patent for rendering molybdenum ductile, resulting in applications such as a heating element for high-temperature furnaces and as a support for tungsten-filament light bulbs; oxide formation and degradation necessitate that molybdenum is physically sealed or held in an inert gas. In 1913 Frank E. Elmore established a froth flotation process to recover molybdenite from ores; flotation is still the primary isolation process.
During the First World War, demand for molybdenum spiked; it was applied both in armor plating and as a substitute for tungsten in high-speed steels. Some British tanks were protected by 75 mm manganese steel plating, but this was shown to be ineffective. The manganese steel plates were substituted with much lighter 25 mm molybdenum steel plates permitting higher speed, greater maneuverability, and better protection. The Germans likewise used molybdenum-doped steel for heavy artillery, for example, in the superheavy howitzer nicknamed Big Bertha (42 cm kurze Marinekanone 14 L/12 (short naval cannon), or Minenwerfer-Gera¨t), as traditional steel melts at the temperatures produced by the propellant of the one-ton shell. After the war, demand fell until metallurgical developments permitted wide-ranging development of peacetime applications. In the Second World War molybdenum once more became strategically important as a replacement for tungsten in steel alloys.
- Related articles
- Related Qustion
- Industrial Applications and Uses of Molybdenum Sep 27, 2019
Molybdenum is largely used in steel industry.Its compounds are widely used incoloring agents, solid lubricants and ascatalysts. In the form of ferromolybdenum for manufg special steels for tools, boiler plate, rifle barrels.
- Industrial Preparation of Molybdenum Metal Sep 27, 2019
High-purity molybdenum metal powder is prepared by reducing either pure molybdenum trioxide or ammonium dimolybdate with pure hydrogen at 600°C but not higher in order to prevent sintering and caking.
- General Properties of Molybdenum Sep 27, 2019
Molybdenum is a hard, moderately dense (10,220 kg.m–3) refractory metal with a high melting point (m.p. 2621.85°C). Its freshly exposed surfaces are silvery white, but when tarnished the metal acquires a steel-gray color.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringGallium has a silvery blue appearance. It has a low melting point at 29.7646℃ and forms a liquid state that is denser than the solid.....
May 28,2024APIMolybdenum
7439-98-7You may like
- Molybdenum
-

- $0.00 / 25KG
- 2025-06-27
- CAS:7439-98-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg
- Molybdenum
-

- $25.00 / 1kg
- 2025-06-20
- CAS:7439-98-7
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 20 tons
- Molybdenum
-

- $3.60 / 1kg
- 2025-06-16
- CAS:7439-98-7
- Min. Order: 1kg
- Purity: ≥99%
- Supply Ability: 3000tons/month