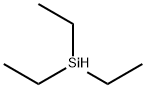
How does the reduction mechanism of triethylsilane and trans fatty acids work?
May 8,2024
Triethylsilane is a commonly used reducing agent in organic reactions for a wide range of functional groups, including alcohols, aldehydes, ketones, and amides. It is particularly suitable for the reduction of carbonyl and imine functional groups. It is usually used in combination with trifluoroacetic acid (TFA) as a co-catalyst. The reduction mechanism of triethylsilane and TFA involves the transfer of hydride ions from the silane to the substrate, resulting in the formation of a silylene ether intermediate. This intermediate can then react with TFA to form a stable carbon ion, which can then be protonated to produce the reduction product. In the reduction reaction, the triethylsilane first needs to be activated, which is usually achieved by sodium or potassium hydroxide. During the activation process, the silicon-hydrogen bonds in the triethylsilane are broken, which enhances its reducing ability.
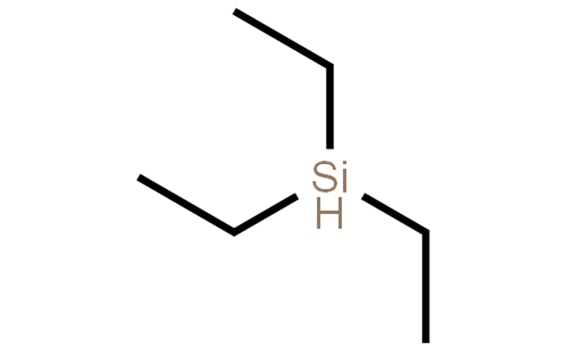
- Related articles
- Related Qustion
- The Versatile Applications and Characteristics of Triethylsilane in Modern Chemistry May 22, 2024
Triethylsilane, a colorless and flammable organosilicon compound, the chemical community due to its unique properties and wide-ranging applications.
- Triethylsilane: General description,Application and Production Apr 28, 2023
Triethylsilane is colorless and transparent liquid, soluble in non-polar solvents and flammable.
- Applications of Triethylsilane Nov 8, 2019
Triethylsilane serves as an exemplar for organosilicon hydride behavior as a mild reducing agent.
Naphthalene is a white crystalline volatile solid in the form of flakes, cakes or powder with a strong coal tar odour.....
May 8,2024APITetrahydro-4H-pyran-4-one, pivotal in drug synthesis, aids in crafting potent histamine-3 antagonists for cognitive disorders, showcasing significant medicinal potential.....
May 9,2024APITriethylsilane
617-86-7You may like
- Triethylsilane
-

- $0.00 / 1kg
- 2024-07-12
- CAS:617-86-7
- Min. Order: 1kg
- Purity: 99
- Supply Ability: 20 tons
- Triethylsilane
-

- $0.00 / 1kg
- 2024-06-18
- CAS:617-86-7
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 50000kg
- Triethylsilane
-

- $120.00 / 1kg
- 2024-05-21
- CAS:617-86-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10ton