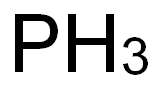Is phosphorus harmful to health? What are the food sources?
May 28,2024
Phosphorus has a concentration in the earth's crust of about 0.1% by weight. It is not found free in nature but is widely found in many minerals, usually as phosphates. Inorganic phosphate rock, which is partially made of apatite (a group of minerals being, generally, pentacalcium triorthophosphate fluoride (hydroxide),Ca5(PO4)3(F,OH)), is today the principal commercial source of this element. About 50% of the worldwide phosphorus reserves are in the Arab nations.
Major minerals
In total there are just under 700 minerals containing P, the majority of them in the form of phosphates. Within the class of the elements, a number of phosphides can be found, such as barringerite ((Fe,Ni)2P) and nickelphosphide ((Ni,Fe)3P). The only halide containing P is bøggildite (Na2Sr2Al2PO4F9).
Similarly, the only oxide is fritzscheite (Mn(UO2)2(PO4,VO4)210H2O). The largest group of minerals is found under the phosphates with more than 500 different minerals. Some of the best-known of these include minerals such as amblygonite (LiAl(PO4)F), augelite (Al2(PO4)(OH)3) , autunite (Ca(UO2)2(PO4)211H2O), brazilianite (NaAl3(PO4)2(OH)4), cacoxenite (Fe3124AlO6(PO4)17(OH)1275H2O) etc.

Fig Autunite
Electronic configuration
Phosphorus is a solid nonmetal at room temperature and a poor conductor of heat and electricity. It belongs to group 15 and has the electronic configuration [Ne]3s23px13py13pz1. It has three unpaired electrons just like nitrogen above it. However, P has available low-lying vacant 3d orbitals and this accounts for the predominant oxidation states III and V in phosphorus chemistry. This allows for valency expansion where P is bonded to 5 or 6 atoms and is surrounded by 10 or 12 pairs of electrons, in exception to the octet rule. Thus, PF5 or PF6 are known but the analogous NF5 or NF6 have not been prepared.
Food sources
The main food sources for phosphorus are the same as those containing protein, although proteins do not contain phosphorus. For example, milk, meat, and soya typically also have phosphorus. As a rule, if a diet has sufficient protein and calcium, the amount of phosphorus is probably sufficient.
Health hazards
People can be exposed to phosphorus in the workplace by inhalation, ingestion, skin contact, and eye contact. The Occupational Safety and Health Administration (OSHA) has set the phosphorus exposure limit (Permissible exposure limit) in the workplace at 0.1 mg/m3 over an 8-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set a Recommended exposure limit (REL) of 0.1 mg/m3 over an 8-hour workday. At levels of 5 mg/m3, phosphorus is immediately dangerous to life and health.
- Related articles
- Related Qustion
- Differences between red and white phosphorus and their applications Dec 18, 2023
White phosphorus is a toxic, waxy solid that can cause severe burns on contact with skin. Red phosphorus is an amorphous, non-toxic powder.
- Phosphorus: Production, application, toxicity and biological role May 6, 2023
White phosphorus is a highly toxic substance by all routes of exposure. Contact of the solid with the skin produces deep painful burns, and eye contact can cause severe damage.
- Phosphorus-Hazard and Toxicity Sep 5, 2019
White phosphorus is a highly toxic substance by all routes of exposure. Contact of the solid with the skin produces deep painful burns, and eye contact can cause severe damage. Ingestion of phosphorus leads (after a delay of a few hours) to
1,7-Dimethylxanthine is a naturally occurring alkaloid compound that can enhance alertness and reduce drowsiness.....
Feb 27,2025APIMolybdenum is the 54th most abundant element in the Earth’s crust. Molybdenum is mined as a principal ore, while also being recovered as a by-product of copper and tungsten mining.....
May 28,2024Inorganic chemistryPhosphorus
7723-14-0You may like
- Phosphorus
-

- $0.00 / 10kg
- 2024-12-17
- CAS:7723-14-0
- Min. Order: 10kg
- Purity: 99% purity
- Supply Ability: 1000 kg
- Phosphorus
-

- $9.00 / 1KG
- 2024-01-08
- CAS:7723-14-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000tons
- Phosphorus
-

- $0.00 / 1ton
- 2023-09-14
- CAS:7723-14-0
- Min. Order: 1ton
- Purity: 99.99
- Supply Ability: 1000000tons