Maleic acid: uses and Toxicity
Aug 26,2024
Introduction
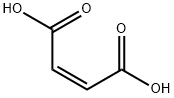
Maleic acid is a linear four-carbon molecule with carboxylate groups on both ends and a double bond between the central carbon atoms. The anhydride of maleic acid is a cyclic molecule containing five atoms. Although the reactivity of maleic anhydride is similar to other cyclic anhydrides, the products of maleylation are much more unstable toward hydrolysis, and the site of unsaturation lends itself to additional side-reactions.

Maleic acid is a colourless crystalline solid having a faint odour. It is combustible, though it may take some effort to ignite. It is soluble in water. It is used to make other chemicals and for dyeing and finishing naturally occurring fibres.
Uses
Maleic acid (MA) is a carboxylic acid with two functional groups. In early studies, it was found that MA can polymerize in the presence of free radical initiators and SHP to form polymaleic acid (PMA). And catalyzed by SHP, it forms a cyclic anhydride and reacts with -OH on the fibers to form an ester cross-linking, which imparts an excellent level of wrinkle resistance for cotton fabrics. Based on this finding, the synthesis of maleic acid-based copolymers for the anti-wrinkle treatment of cotton fabrics has gained some feasibility.
Maleic acid is manufactured by hydrolysis of maleic anhydride. There are, however, few applications for maleic acid, the major industrial use being the isomerization into fumaric acid. Fumaric acid occurs naturally in many plants and is named after Fumaria officinalis, a climbing annual plant from which it was first isolated. Currently, industrial synthesis of fumaric acid is mostly based on catalytic isomerization of maleic acid in aqueous solutions. Malic acid (hydroxybutanedioic acid, HOOCCH(OH)CH2COOH) is produced by the hydration of either fumaric or maleic acid. It is also a natural acid in green apples and other unripe fruits.
Toxicity
In water, maleic anhydride rapidly hydrolyzes to form maleic acid. It was reported that maleic acid is a potent inhibitor of several enzyme systems in vitro. Furthermore, in vivo effects have been observed in the kidney. Other studies have found that treating rats with maleic acid can lead to atypical renal tubular epithelial cells, aminoaciduria, glycosuria, and phosphaturia. There are few studies on the toxicological effects of maleic acid on humans. Tuncel et al. reported that the cytotoxicity of maleic acid to human renal cells is time- and concentration-dependent. The European Food Safety Authority (EFSA) has set the TDI (tolerable daily intake) of maleic acid as 0.5 mg/kg bw. Maleic acid can be formed in food when malic acid or fumaric acid is converted at high temperatures during the Maillard reaction. For example, Ohiokpehai's report from the University of Surrey in the U.K. stated that maleic acid had been found in roasted coffee beans, and maleic acid had also been found in honey at 2.6 or 4.6 ppm. In plastic food packages, maleic acid and maleic anhydride could be used as additives, polymer production aids, and monomers at the total specific migration limit [SML (T)] of 30 mg/kg determined by the maleic acid content. In brief, high concentrations of maleic acid have been shown to induce Fanconi syndrome, whereas low concentrations of maleic acid appear not to be toxic to humans in current research.
References
[1] Maleic Acid - an overview | ScienceDirect Topics https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/maleic-acid
[2] Maleic Acid | C4H4O4 | CID 444266 - PubChem (nih.gov) https://pubchem.ncbi.nlm.nih.gov/compound/Maleic-Acid
- Related articles
- Related Qustion
- What is maleic acid used for? Nov 9, 2022
Maleic acid is used in making polyesters for fibre-reinforced laminated moldings and paint vehicles, and in the manufacture of fumaric acid and numerous additional chemical products.
Testosterone propionate enhances BM-MSCs' proliferation and viability, while also reducing leukemia cell viability, through inflammatory signaling modulation, promising in cancer therapy.....
Nov 4,2024APICalcium propionate is an organic salt formed by the reaction between calcium hydroxide and propionic acid and has the molecular formula (CH3CH2COO)2Ca.....
Aug 26,2024Food AdditivesMaleic acid
110-16-7You may like
- Maleic acid
-

- $77.00 / 5g
- 2024-11-04
- CAS:110-16-7
- Min. Order:
- Purity: ≥98%
- Supply Ability: 10g
- Maleic acid
-

- $45.00/ kg
- 2024-11-04
- CAS:110-16-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Maleic acid
-

- $0.00 / 25Kg/Bag
- 2024-11-04
- CAS:110-16-7
- Min. Order: 25KG
- Purity: 99%-101%
- Supply Ability: 20 tons