The review of sodium bicarbonate
Mar 9,2022
Background
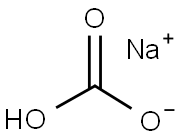
Sodium bicarbonate, commonly known as baking soda, has the chemical formula NaHCO3. Its aliases are not as many as soda. In addition to baking soda, there are only sodium bicarbonate and sodium acid carbonate. Usually white crystalline powders(picture 1), odorless, easily soluble in water. It decomposes slowly in moist or hot air, producing carbon dioxide. It is easy to decompose when heated. When heated to 270 °C, it is completely decomposed, and the decomposition products are sodium carbonate, water and carbon dioxide.

Picture 1 Sodium bicarbonate powders
Differences between sodium carbonate and sodium bicarbonate
First of all, it can be seen from their chemical formula that sodium carbonate is heavier. Under the same weight, sodium bicarbonate has more weight than sodium carbonate. From their appearance, sodium carbonate is mainly white powder, white and bright, and sodium bicarbonate is mainly white crystal, white and dark. Sodium bicarbonate is unstable, so it will decompose in the air, but sodium bicarbonate just absorbs water and clumps. For the same weight of aqueous solution, sodium carbonate is more alkaline, and sodium bicarbonate is only weakly alkaline. This is well understood because sodium bicarbonate is hydrolyzed to form sodium carbonate.
The role of sodium bicarbonate in medicine
Sodium bicarbonate tablets, also known as baking soda tablets, are an antacid that can quickly neutralize stomach acid and relieve symptoms of hyperacidity or heartburn after oral administration, but the effect is weak and the duration is short. For gout patients, soda tablets are more commonly used drugs. They are cheap and easy to buy. They can alkalize urine, assist uric acid excretion, and reduce the risk of kidney uric acid stones.
The dangers of overuse of sodium bicarbonate
1. Effects on the digestive system: Long-term use of sodium bicarbonate will neutralize gastric acid, which will inevitably affect digestion, resulting in bitter mouth, loss of appetite and nausea, and will produce a large amount of carbon dioxide to cause belching and secondary Increased gastric acid secretion.
2. Effects on the cardiovascular system: Long-term use of sodium bicarbonate will lead to excessive sodium load in the body, resulting in water and sodium retention, causing edema, which will increase the load on the heart, affect heart function, and make blood vessels brittle. For patients with cardiac insufficiency and high blood pressure, it needs to be used with extreme caution.
3. Effects on the urinary system: Long-term use of sodium bicarbonate will lead to metabolic alkalosis of the kidneys, which is especially unfavorable for people with renal insufficiency. Many people will also experience symptoms such as frequent urination and urgency.
4. Interfere with the absorption of calcium: Sodium bicarbonate (NaHC03) decomposes carbonate CO3 in the body, and CO3 combines with calcium (Ca) to form insoluble calcium carbonate. Due to the presence of a large amount of CO3 in the body and the competitive binding of calcium with the body, it will lead to calcium deficiency and osteoporosis.
Recommendations
Baking soda does relieve gout, but it does not cure gout. It is recommended not to take baking soda when it is not an acute attack of gout. How long a patient with gout or hyperuricemia needs to take baking soda depends on the pH of their urine. The results of the study show that the urine pH value of 6.2-6.9 is conducive to the dissolution and excretion of urate crystals from the urine, and the urine pH value of 6.5-6.8 is the most suitable range. When the pH value is below 6.0, it is necessary to alkalize the urine. When the pH value exceeds 7.0, calcium oxalate and other types of stones are likely to be produced, and the drug should be discontinued. Oral baking soda tablets need to drink plenty of water at the same time to ensure urine output.
Renference
1 Forsythe S M, Schmidt G A. Sodium bicarbonate for the treatment of lactic acidosis[J]. Chest, 2000, 117(1): 260-267.
2 McNaughton L R, Siegler J, Midgley A. Ergogenic effects of sodium bicarbonate[J]. Current sports medicine reports, 2008, 7(4): 230-236.
- Related articles
- Related Qustion
- What are the medicinal functions and benefits of sodium bicarbonate? Jan 3, 2025
Sodium bicarbonate (NaHCO3 or baking soda, abbreviated as SB), is a weak alkaline compound (pH 8.5) and belongs to the class of antacid drugs.
- Baking Powder And Vinegar Reaction Mar 7, 2024
Mixing Baking Powder and vinegar creates a chemical reaction
- The Manufacturing Process and Uses of Baking Soda Mar 4, 2024
Baking soda is a white, water-soluble crystalline solid with an alkaline taste, this article will introduce its manufacturing process and uses.
Epsilon-polylysine (ε-polylysine), a fermentative by-product of Streptomyces albulus , is a polyamino acid (not a protein), comprised exclusively of l-lysine residues linked together by amide bonds. ε-Polylysine is used extensively in Japan....
Mar 8,2022Biochemical EngineeringSilymarin is a botanical extract derived from milk thistle. There are several studies that have evaluated individual components of silymarin in dermatology, and fewer studies that have evaluated the properties.....
Mar 9,2022Plant extractsSodium bicarbonate
144-55-8You may like
Sodium bicarbonate manufacturers
- Sodium bicarbonate
-

- $3.00 / 5kg
- 2025-04-25
- CAS:144-55-8
- Min. Order: 50kg
- Purity: 98.9%
- Supply Ability: 1000
- sodium Bicarbonate
-

- $6.00 / 1kg
- 2025-04-25
- CAS:144-55-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month
- sodium Bicarbonate
-

- $6.00 / 1kg
- 2025-04-25
- CAS:144-55-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month