Vonoprazan Fumarate: Physicochemical Properties and Pharmacokinetics
Sep 2,2024
General Description
Vonoprazan fumarate is a potassium-competitive acid blocker with a high pKa of 9.06, leading to effective accumulation in gastric parietal cells. Vonoprazan fumarate exhibits superior potency in inhibiting H+,K+-ATPase, being significantly more potent than other drugs in its class. Following oral administration, vonoprazan demonstrates rapid absorption, reaching peak plasma concentration within 1.5 to 2.0 hours, with a mean oral clearance of 97.5 L/h and a terminal half-life of 7.7 hours. Vonoprazan fumarate undergoes extensive metabolism primarily via CYP3A4 and CYP2B6, with minimal unchanged drug excreted in urine, suggesting active renal transport involvement.

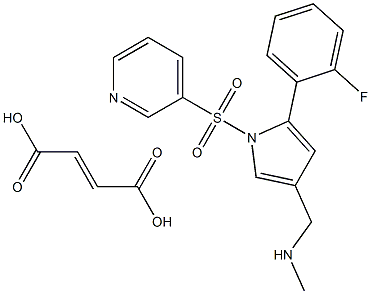
Figure 1. Vonoprazan fumarate
Physicochemical Properties
Vonoprazan fumarate, an acid-resistant pyrrole derivative, is characterized by its significant physicochemical properties. It has a higher pKa value of 9.06 compared to other potassium-competitive acid blockers (P-CABs) like SCH28080, which has a pKa of 5.6. This higher point-positive charge leads to greater accumulation of vonoprazan fumarate in the canalicular space of gastric parietal cells. The drug is chemically designed as 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine fumarate (TAK-438). Its interaction with the H+,K+-ATPase is strictly potassium-competitive and reversible, with a Ki of 10 nM at pH 7.0, reflecting its robust binding affinity. 1
Potency and Duration
Vonoprazan fumarate demonstrates superior potency in inhibiting H+,K+-ATPase compared to other P-CABs and proton pump inhibitors (PPIs). It is 10 times more potent than SCH28080 and 350 times more potent than lansoprazole in porcine gastric microsomes at pH 6.5. The half maximal inhibitory concentrations (IC50) for vonoprazan are 0.019 nM, showcasing its high efficacy. Additionally, vonoprazan fumarate has a slower rate of dissociation from isolated ATPase, with a half-life of 7.5 hours in 20 mM KCL, which is close to physiological concentrations. This extended duration contributes to its more potent and prolonged antisecretory effects observed in animal studies. 1
Pharmacokinetics
Absorption and Bioavailability
Vonoprazan Fumarate, a novel potassium-competitive acid blocker, exhibits distinct pharmacokinetic properties. Following oral administration, vonoprazan demonstrates rapid intestinal absorption, as evidenced by studies in Japan and the UK involving healthy subjects. After single doses of vonoprazan at varying strengths (10, 15, 20, 30, and 40 mg), maximum plasma concentration (Cmax) increased in a largely dose-proportional manner, ranging from approximately 10 to 60 ng/mL, with a time to reach Cmax of 1.5–2.0 hours. Furthermore, upon repeated dosing over seven days, vonoprazan showed nearly complete accumulation by the third day, demonstrating stable pharmacokinetics with accumulation factors for Cmax and area under the curve (AUC0–inf) between 1.14 and 1.32. Despite the rapid absorption, the oral bioavailability of vonoprazan in humans remains largely unknown due to the absence of intravenous studies. A mass balance study indicated that while 67% and 31% of administered 14C-vonoprazan was recovered in urine and feces, respectively, only a small fraction was unchanged, suggesting extensive metabolism occurs post-absorption. 2
Distribution and Protein Binding
The distribution characteristics of vonoprazan are noteworthy, given that precise plasma concentration-time data following intravenous administration is lacking. According to a study involving healthy male subjects, the mean apparent volume of distribution for vonoprazan Fumarate was recorded at 1056 ± 263 L after a 20 mg oral dose. This suggests that the true volume of distribution is likely greater than 84 L, with a significant accumulation of vonoprazan occurring in the gastric parietal cells due to its basic nature and pKa of approximately 9.0. This allows for higher concentrations in the gastric secretory canaliculi, exceeding plasma concentrations by more than 1000 times. Additionally, the plasma protein binding of vonoprazan was found to be low, with unbound fractions ranging from 12% to 15% across various concentrations. As the plasma concentrations of vonoprazan typically remain below therapeutic levels, it is unlikely that significant saturation of protein binding occurs, indicating a favorable pharmacokinetic profile. 2
Elimination and Metabolism
The elimination and metabolic pathways of vonoprazan Fumarate highlight its unique pharmacokinetic features. The mean oral clearance of the drug is reported to be 97.5 ± 30.1 L/h, with a terminal half-life averaging at 7.7 hours. In vitro studies suggest that vonoprazan is primarily metabolized by the cytochrome P450 enzymes CYP3A4 and CYP2B6, among others, with none of the metabolites exhibiting pharmacological activity. Notably, vonoprazan shows direct inhibitory effects on these CYP isozymes, although the therapeutic plasma concentrations are lower than the inhibitory values, implying minimal clinical significance in drug interactions. Furthermore, only 4% of the administered vonoprazan dose is recovered as unchanged drug in urine, with a mean renal clearance suggesting involvement of active transport mechanisms, exceeding glomerular filtration rates, thereby complicating the systemic clearance assessments. These features indicate that while metabolism and renal excretion play roles in the pharmacokinetics of vonoprazan, further studies are warranted to fully understand the contributions of these pathways. 2
Reference
1. Otake K, Sakurai Y, Nishida H, et al. Characteristics of the Novel Potassium-Competitive Acid Blocker Vonoprazan Fumarate (TAK-438). Adv Ther. 2016; 33(7): 1140-1157.
2. Echizen H. The First-in-Class Potassium-Competitive Acid Blocker, Vonoprazan Fumarate: Pharmacokinetic and Pharmacodynamic Considerations. Clin Pharmacokinet. 2016; 55(4): 409-418.
- Related articles
- Related Qustion
Lurasidone hydrochloride is clinically beneficial in treating patients with schizophrenia and bipolar depression.....
Nov 8,2024APIDesonide, a nonfluorinated corticosteroid, treats conditions like psoriasis and atopic dermatitis with enhanced absorption in inflamed or moisturized skin, improving therapeutic outcomes.....
Sep 2,2024APIVonoprazan fumarate
1260141-27-2You may like
Vonoprazan fumarate manufacturers
- Vonoprazan Fumarate
-

- $0.00 / 25KG
- 2024-11-08
- CAS:1260141-27-2
- Min. Order: 2KG
- Purity: 99% min/GMP/PMDA/DMF
- Supply Ability: 20tons
- Vonoprazan Fumarate
-

- $0.00 / 1g
- 2024-11-08
- CAS:1260141-27-2
- Min. Order: 1g
- Purity: More Than 99%
- Supply Ability: 50kg/Month
- Vonoprazan fumarate
-

- $40.00 / 500mg
- 2024-11-07
- CAS:1260141-27-2
- Min. Order:
- Purity: 99.16%
- Supply Ability: 10g