N-МЕТИЛФОРМАМИД химические свойства, назначение, производство
Химические свойства
N-methylformamide is a clear colorless liquid with a slight amine odor. It is a water-soluble organic solvent. As an adjuvant antineoplastic agent, N-methylformamide depletes cellular glutathione, a key molecule involved in the antioxidation of reactive oxygen species (ROS) and other free radicals, thereby enhancing ionizing radiation-induced DNA cross-linking in and terminal differentiation of tumor cells. (NCI04)
Использование
N-Methylformamide is used in amidation or transamidation chemical reactions where formamide is insufficient.
Определение
ChEBI: N-methylformamide is a member of the class of formamides having a N-methyl substituent. It is functionally related to a formamide.
Методы производства
N-Methylformamide (NMF) can be synthesized by: (1) reacting methylamine, carbon monoxide, methanol and a small amount of potassium acetate at 250 atm and 160°C; (2) heating methylamine with carbon monoxide and some sodium ethoxide in ethanol at 150 atm; (3) treating methylformate with methylamine and methanol; (4) reacting hexamethylenetetramine with formamide and hydrogen in the presence of Raney nickel at 130-145°C (Beilstein's Handbuch, 1977).
Общее описание
N-Methylformamide (NMF), a formamide derivative, is widely employed as an organic solvent. NMF molecule contains C=O and N-H groups, so it can act as a proton donor and acceptor. Due to its important physical properties such as high dielectric constant, high solubility, low conductivity and amphiprotic nature, it is suitable for use as a separation medium in capillary electrophoresis studies.
NMF is closely related to other formamides, notably formamide and dimethylformamide (DMF). However, industrial use and production of NMF are far less than for either of these other formamides. DMF is favored over NMF as a solvent due to its greater stability. Annual production of NMF can be assumed to be significantly less than the production of either formamide (100,000 tons) or DMF (500,000 tons).
Реакции воздуха и воды
Water soluble.
Профиль реактивности
N-Methylformamide is incompatible with benzene sulfonyl chloride. N-Methylformamide is also incompatible with strong oxidizing agents, acids, bases and acid chlorides. N-Methylformamide may react with chlorine, bromine, nitrates, nitric acid, triethylaluminum, potassium permanganate, chromic acid, chromic anhydride, chromium trioxide, borohydrides, hydrides, thionyl chloride, metallic sodium, phosphorus trioxide, diborane, (octafluoroisobutyrate + sodium nitrite) and (perchloryl fluoride + potassium methyl 4,4-dinitrobutyrate).
Угроза здоровью
SYMPTOMS: Symptoms of exposure to this compound include irritation of the mucous membranes and upper respiratory tract. Other symptoms include liver damage, eye irritation with discomfort, tearing or blurring of vision, skin irritation with discomfort or rash, abnormalities of liver function with jaundice, temporary nervous system depression with anesthetic effects such as dizziness, headache, confusion, incoordination and loss of consciousness.
ACUTE/CHRONIC HAZARDS: N-Methylformamide may be absorbed through the skin and cause skin irritation. It may also irritate the eyes, mucous membranes and upper respiratory tract. When heated to decomposition it emits toxic fumes of carbon monoxide, carbon dioxide and nitrogen oxides. (NTP, 1992)
Пожароопасность
N-Methylformamide is combustible.
Промышленное использование
NMF possesses excellent solvent properties that are similar to those of dimethylformamide. However, NMF appears to be much less important as an industrial solvent than dimethylformamide.
Профиль безопасности
Moderately toxic by
ingestion, intraperitoneal, intravenous,
intramuscular, and subcutaneous routes. An
experimental teratogen. Experimental
reproductive effects. An eye irritant. A very
dangerous fire hazard when exposed to heat
or flame. Violent reaction with benzene
sulfonyl chloride. When heated to
decomposition it emits toxic fumes of NOx.
Методы очистки
Dry it over molecular sieves for 2days, then distil it under reduced pressure through a column packed with glass helices. Fractionally crystallise it by partial freezing and the solid portion is distilled in a vacuum. [Beilstein 4 IV 170.]
Оценка токсичности
N-Methylformamide (NMF) is a metabolite of dimethylformamide (DMF), a solvent with wide applications in the chemical industry.Pregnant rats and rabbits were dosed once daily by gavage on Gestation Days 6-15 and 6-18, respectively. Doses for rats were 0, 1, 5, 10, or 75 mg/kg; doses for rabbits were 0, 5, 10, or 50 mg/kg. No treatment-related maternal deaths or clinical signs occurred in either species. Body weight gain and food consumption were depressed in rats given 75 mg/kg and rabbits given 50 mg/kg. Fetal viability was reduced at both rabbits and rats. The lowest-observed-adverse-effect levels for maternal and developmental toxicity in the rat and rabbit were 75 and 50 mg/kg, respectively. he no-observed-adverse-effect level for maternal and developmental toxicity in the rat and rabbit was 10 mg/kg[1].
Структура и конформация
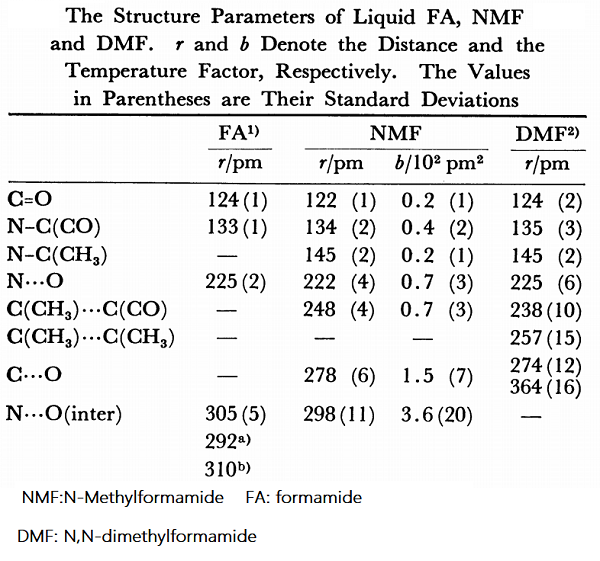
The liquid structure of N-methylformamide (NMF) has been investigated using the X-ray diffraction method and the ab initio MO-SCF method. The structure parameters within a molecule were obtained as follows: C=O: 122(1) pm, C(methyl)–N: 145(2) pm, C(carbonyl)–N: 134(2) pm, N···O: 222(4) pm, C(methyl)···O: 278(6) pm. The intermolecular hydrogen-bonded N···O distance was estimated to be 298(11) pm. A linear and flexible chain structure was proposed for the liquid structure of NMF based on scattered intensity data by the X-ray diffraction method and of interaction energies and geometries of hydrogen-bonded NMF molecules calculated by the ab initio MO calculations[1].
N-МЕТИЛФОРМАМИД препаратная продукция и сырье
сырьё
препарат