Диметиламин химические свойства, назначение, производство
Описание
Dimethylamine is a colourless flammable gas at room temperature. It has a pungent, fishy, or ammonia-like odour at room temperature and is shipped and marketed in compressed liquid form. It is very soluble in water and soluble in alcohol and ether. It is incompatible with oxidising materials, acrylaldehyde, fluorine, maleic anhydride, chlorine, or mercury. Dimethylamine is a precursor to several industrially important compounds. For instance, it used in the manufacture of several products, for example, for the vulcanisation process of rubber, as detergent soaps, in leather tanning, in the manufacture of pharmaceuticals, and also for cellulose acetate rayon treatment.
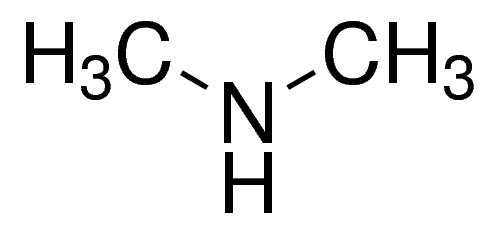
dimethylamine structure
Химические свойства
Dimethylamine reacts readily with acids to produce salts due to the presence of the unshared electron pair on the nitrogen atom. Similarly, dimethylamine reacts with acid anhydrides, halides, and esters, with CO2 or CS2, or with isocyanic or isothiocyanic acid derivatives. It can also react with nitrite, especially under acidic conditions, and possibly nitrogen oxides (Iqbel 1986) to form N-nitrosodimethylamine, a potent carcinogen in various animal species and a suspect human carcinogen (ATSDR 1989; Scanlan 1983; Zeisel et al 1988). N-Nitrosodimethylamine also can be formed upon storage of aqueous dimethylamine solutions or formulations of the dimethylamine salts of the herbicides 2,4D and MCPA (Wigfield and McLenaghan 1987a,b). Dimethylamine also can be nitrosated photochemically in aqueous solutions containing nitrite with the reaction occurring most readily at alkaline pH (Ohta et al 1982).
Физические свойства
Clear, colorless liquid or gas with a strong, ammonia-like odor. Odor threshold concentrations of
33 ppbv and 47 ppbv were experimentally determined by (Leonardos et al., 1969) and Nagata and
Takeuchi (1990), respectively.
Использование
Dimethylamine is used in the manufactureof N-methylformamide, N-methylacetamide,and detergent soaps; in tanning; and as anaccelerator in vulcanizing rubber. It is commercially sold as a compressed liquid intubes or as a 33% aqueous solution..
Определение
ChEBI: A secondary aliphatic amine where both N-substituents are methyl.
Реакции воздуха и воды
Highly flammable. Water soluble.
Профиль реактивности
DIMETHYLAMINE is a base, neutralizing acids in exothermic reactions, and a reducing agent. Dimethylamine is temperature sensitive. Reacts vigorously with mercury and chlorine . Reacts violently with strong oxidizing agents and attacks copper and copper compounds [Handling Chemicals Safely, 1980 p. 123]. Reacts with hypochlorites to give N-chloroamines, some of which are explosives when isolated [Bretherick, 1979 p. 108].
Опасность
Dimethylamine is an irritant, with a TLV of 10 ppm in air. The four-digit UN identification number is 1032. The NFPA 704 designation is health 3, flammability 4, and reactivity 0. The primary uses are in electroplating and as gasoline stabilizers, pharmaceuticals, missile fuels, pesticides, and rocket propellants.
Угроза здоровью
Dimethylamine is a strong irritant to the eyes,skin, and mucous membranes. Spill of liquidinto the eyes can cause corneal damage andloss of vision. Skin contact with the liquidcan produce necrosis. At sublethal concentra tions, inhalation of dimethylamine producedrespiratory distress, bronchitis, pneumonitis,and pulmonary edema in test animals. Theacute oral toxicity was moderate, greater thanfor monomethylamine.
LC50 value, inhalation (rats): 4540 ppm/6 hLD50 value, oral (mice): 316 mg/kg
Buckley and coworkers (1985) have investigated the inhalation toxicity of dimethylamine in F-344 rats and B6C3F1 mice.Animals exposed to 175 ppm for 6 h/day,5 days/week for 12 months showed significant lesions in the nasal passages. Rats developed more extensive olfactory lesions thandid mice. The study indicated that olfactory sensory cells were highly sensitive todimethylamine. Even at a concentration of10 ppm, the current threshold limit value,the rodents developed minor lesions fromexposure.
Пожароопасность
FLAMMABLE. Flashback along vapor trail may occur. May explode if ignited in an enclosed area. Vapors are eye, skin and respiratory irritants.
Промышленное использование
Dimethylamine is used as an accelerator in vulcanizing rubber, as an antiknock
agent for fuels, in photography, as a plasticizer, ion exchange agent, as an acid gas
absorbent, a flotation agent, a dehairing agent in the tanning of leather and in
electroplating (HSDB 1989; Sax and Lewis 1987; Windholz et al 1983). Dimethylamine
also serves as the base for a large number of commercial products
including detergent soaps, dyes, pharmaceuticals, textile chemicals, surfactants
and in the manufacture of unsymmetrical dimethylhydrazine (used in missile
fuels), the solvent dimethylacetanilide and in the synthesis of dimethylformamide,
one of the most commonly used organic solvents. Usage of dimethylamine in 1972
was estimated at 50% for production of dimethylformamide and dimethylacetamide
(used as spinning solvents for acrylic fibers), 15% as an intermediate in the
preparation of the surfactant laurel dimethylamine oxide, 15% as an intermediate
for rubber chemicals (including thorium accelerators), and 20% for other applications
including the production of unsymmetrical dimethylhydrazine in rocket fuels
and the dimethylamine salt of 2,4-dichlorophenoxyacetic acid (HSDB 1989). U.S.
production and sales of dimethylamine in 1985 was 65.9 million pounds.
Профиль безопасности
Poison by ingestion.
Moderately toxic by inhalation and
intravenous routes. Mutation data reported.
An eye irritant. Corrosive to the eyes, skin,
and mucous membranes. A flammable gas.
When heated to decomposition it emits
toxic fumes of Nx,. Incompatible with
acrylddehyde, fluorine, and maleic
anhydride
Канцерогенность
In a 2 year inhalation study in male F344 rats exposed to
175 ppm, no evidence of carcinogenicity was observed, and
in addition, despite severe tissue destruction in the anterior
nose following a single 6 h exposure, the nasal lesions
exhibited very little evidence of progression, even at
2 years of exposure. The authors concluded that this
indicated possible regional susceptibility to DMA toxicity or
a degree of adaptation by the rat to continued DMA exposure.
A detailed evaluation of mucociliary apparatus function
and response to alterations of nasal structure was presented
by the authors.
хранилище
Dimethylamine should be stored in a cool, dry, well-ventilated area in tightly sealed containers
that are labeled in accordance with OSHA’s Hazard Communication Standard [29
CFR 1910.1200]. Containers of dimethylamine should be protected from physical damage
and ignition sources, and should be stored separately from oxidizing materials, acrylaldehyde,
fl uorine, maleic anhydride, chlorine, and mercury. Outside or detached storage is
preferred. If stored inside, a standard flammable liquids cabinet or room should be used.
Ground and bond metal containers and equipment when transferring liquids. Empty
containers of dimethylamine should be handled appropriately.
Методы очистки
Dry dimethylamine by passage through a KOH-filled tower, or by standing with sodium pellets at 0o during 18hours. [Beilstein 4 IV 128.]
Меры предосторожности
During handling of dimethylamine, workers should use proper fume hoods, personal
protective clothing and equipment, avoid skin contact, and use gloves, sleeves, and encapsulating suits. Dimethylamine is extremely flammable and may be ignited by heat,
sparks, or open flames. Liquid dimethylamine will attack some forms of plastic, rubber,
and coatings and is flammable. The vapors of dimethylamine are an explosion and poison
hazard. Containers of dimethylamine may explode in the heat of a fi re and require proper
disposal. Workers should use dimethylamine with adequate ventilation and containers
must be kept properly closed.
Диметиламин препаратная продукция и сырье
сырьё
препарат
N-Methylmethanamine 2,4-dichlorophenoxyacetate
6-(Dimethylamino)purine
Methyl cyclopentenolone
DIFENOXURON
Топотекан
3-диметиламино-1-пропанол
Potassium oleate
Этил 3-(N,N-диметиламино)акрила
Гидрохлорид Хлорпромазин
N,N-ДИМЕТИЛЦИКЛОГЕКСИЛАМИН
camazepam
Гексадецилтриметиламмоний хлорид
Диметилдитиокарбамат мышьяка
Insecticide double agent
3-Chloro-1-(N,N-dimethyl)propylamine
3-DIMETHYLAMINOPROPIONIC ACID
2-бром-N,N-диметилбензиламин
Диаллилдиметиламмоний хлорид
Dye-fixing agent,no formaldehyde
Диэтил 3,5-ди-трет-бутил-4-гидроксибензилфосфат
Polyquaternium-7
Dimethylaminopropionitrile
N,N,N',N'-TETRAMETHYL-2-BUTENE-1,4-DIAMINE
2-Hydrazinyl-N,N-dimethylacetamide
3-(CHLOROMETHYL)-N,N-DIMETHYLBENZENESULFONAMIDE
Доксепин
N, N-ДИМЕТИЛДИТИОКАРБАМИНОВАЯ КИСЛОТА
диметил(2-феноксиэтил)амин
Дифенгидрамин
1,1-диметилвигuanиде гидрохлорид
Light stabilizer 2002
GRамин
5-Хлор-1-метилимидазола
N,N-диметилакриламид
4-AMINO-N,N-DIMETHYLBENZYLAMINE
Трис (диметиламинометил) фенол
Диметилдиоктадециламмоний бромид
3-диметиламинопропилхлорид гидрохлорид
Алтретамин
2,2'-Тиобизэтиламин