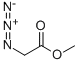
Azidoacetic acid methyl ester
|
- ₹0
- Product name: Azidoacetic acid methyl ester
- CAS: 1816-92-8
- MF: C3H5N3O2
- MW: 115.0907
- EINECS:
- MDL Number:MFCD01311034
- Synonyms:Azidoacetic acid methyl ester;2-Azido-acetic acid Methyl ester;Acetic acid, 2-azido-, Methyl ester;Methyl 2-azidoacetate 97%;Methyl 2-azidoacetate - M3461;methyl 3-diazo-3-azaprop-3-enoate
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Boiling point :35 °C
Density :1.182 g/mL at 25 °C
refractive index :n20/D1.440
Flash point :48℃
Density :1.182 g/mL at 25 °C
refractive index :n20/D1.440
Flash point :48℃
Safety Information
| Symbol(GHS): |
 
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | |||||||||||||||||||||||||||||||||||
| Hazard statements: |
|
|||||||||||||||||||||||||||||||||||
| Precautionary statements: |
|
Description
- Methyl 2-azidoacetate is widely used as a precursor to build triazole derivatives via alkyne-azide click chemistry. Some of the examples include the synthesis of coumarin-triazole derivatives as potential antiplasmodial agents and macrocyclic triazole containing largazole analogs as histone deacetylases-1 (HDAC1) inhibitors.
- It can be used to synthesize various pyrrole derivatives by Knoevenagel condensation as in the synthesis of near-infrared boron dipyrromethene (BODIPY) donors for organic tandem solar cells.
- It can also be used in Hemetsberger-Knittel synthesis of substituted 5-, 6-, and 7-azaindoles.
- Synthesis of highly fluorescent pyreno[2,1-b]pyrroles using methyl 2-azidoacetate as one of the reagents.
Related product price
- AZIDOACETIC ACID
₹5500-8991 - MMTS
₹4643 - ETHYL AZIDOACETATE
₹3200-36597.83






