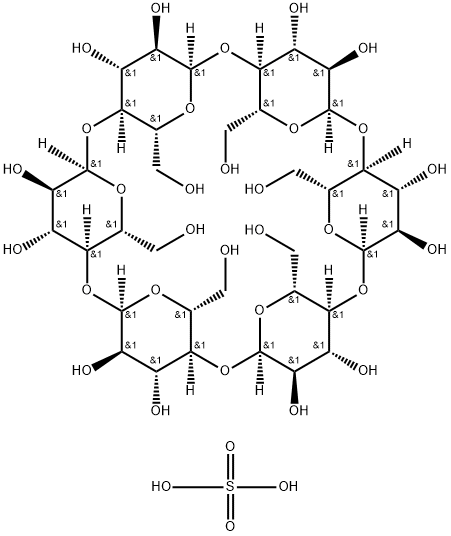
α-Cyclodextrin, sulfated sodium salt
|
- ₹18651.48
- Product name: α-Cyclodextrin, sulfated sodium salt
- CAS: 699020-02-5
- MF: C36H62O34S
- MW: 1070.92
- EINECS:
- MDL Number:MFCD10567418
- Synonyms:ALPHA-CYCLODEXTRIN SULFATED SODIUM SA&;ALPHA-CYCLODEXTRIN, SULFATED, SODIUM SAL T HYDRATE;α-cyclodextrin, sulfated sodium salt hydrate;α-Cyclodextrin, sulfated hydrate sodium salt;alpha-Cyclodextrin hydrate sulfate sodium salt;alpha-Cyclodextrin hydrogen sulfate sodium salt hydrate;alpha-cyclodextrin hydrate sulfate sodium;Alpha cyclo dextrin sulphate
1 prices
Selected condition:
Brand
- Sigma-Aldrich(India)
Package
- 5G
- ManufacturerSigma-Aldrich(India)
- Product number494542
- Product descriptionα-Cyclodextrin, sulfated sodium salt hydrate
- Packaging5G
- Price₹18651.48
- Updated2022-06-14
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 494542 | α-Cyclodextrin, sulfated sodium salt hydrate | 5G | ₹18651.48 | 2022-06-14 | Buy |
Properties
Melting point :190 °C (dec.)(lit.)
solubility :Soluble in water. Insoluble in acetone, methanol, chloroform.
form :Solid powder
color :White to off white
optical activity :[α]23/D +78°, c = 1 in H2O
solubility :Soluble in water. Insoluble in acetone, methanol, chloroform.
form :Solid powder
color :White to off white
optical activity :[α]23/D +78°, c = 1 in H2O
Safety Information
| Symbol(GHS): | ||||||||
|---|---|---|---|---|---|---|---|---|
| Signal word: | ||||||||
| Hazard statements: |
|
|||||||
| Precautionary statements: |
|
Description
The sodium salt of α-Cyclodextrin, sulfated sodium salt is an oligosaccharide commonly employed as a glycoconjugate. It has demonstrated efficacy in the synthesis of glycoproteins, which are proteins with attached carbohydrate chains. Derived from glucose and alpha-cyclodextrin, this oligosaccharide bears a fluorinated methyl group on the 6th carbon atom of its sugar ring. This particular modification enhances its stability in acidic conditions and reduces its vulnerability to hydrolysis by amylases, enzymes responsible for carbohydrate breakdown.More related product prices
37191-69-8 128446-36-6 (2-HYDROXYPROPYL)-BETA-CYCLODEXTRIN 128446-33-3 SODIUM SULFATE DECAHYDRATE Cyclohexapentylose 2-Cyclohexylmandelic acidRelated product price
- 37191-69-8
₹7956.38-18521.58 - 128446-36-6
₹3501-886480.9 - (2-HYDROXYPROPYL)-BETA-CYCLODEXTRIN
₹2592-570012.03






