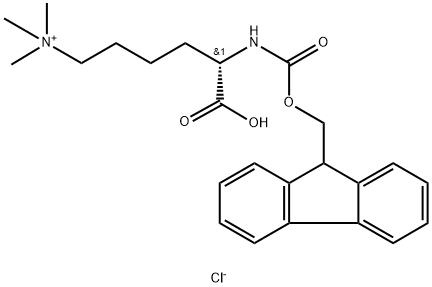
FMOC-LYS(ME3)-OH HCL
|
- ₹16031.83 - ₹65913.43
- Product name: FMOC-LYS(ME3)-OH HCL
- CAS: 201004-29-7
- MF: C24H31ClN2O4
- MW: 446.97
- EINECS:
- MDL Number:MFCD00672333
- Synonyms:N-ALPHA-(9-FLUOROENYLMETHYLOXYCARBONYL)-N-EPSILON-TRIMETHYLAMONIUM-L-LYSINE CHLORIDE;Fmoc-N',N',N'-trimethyl-L-lysine chloride;Fmoc-Lys(Me)3-OH Cl;REF DUPL: (S)-2-(((9H-fluoren-9-yl)methyl9H-fluoren-9-yl)methoxy)carbonylamino)-6-(dimethylamino)hexanoic acid hydrochloride;FMOC-LYSINE(ME)3-OH CHLORIDE;FMOC-LYS(ME)3-OH CHLORIDE;FMOC-LYS(ME3)-OH HCL;FMOC-N-EPSILON-(TRIMETHYL)-L-LYSINE CHLORIDE
2 prices
Selected condition:
Brand
- Sigma-Aldrich(India)
Package
- 500MG
- 2.5G
- ManufacturerSigma-Aldrich(India)
- Product numberF5062
- Product descriptionFmoc-Lys(Me)3-OH Chloride ≥97%
- Packaging500MG
- Price₹16031.83
- Updated2022-06-14
- Buy
- ManufacturerSigma-Aldrich(India)
- Product numberF5062
- Product descriptionFmoc-Lys(Me)3-OH Chloride ≥97%
- Packaging2.5G
- Price₹65913.43
- Updated2022-06-14
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | F5062 | Fmoc-Lys(Me)3-OH Chloride ≥97% | 500MG | ₹16031.83 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | F5062 | Fmoc-Lys(Me)3-OH Chloride ≥97% | 2.5G | ₹65913.43 | 2022-06-14 | Buy |
Properties
storage temp. :-20°C
solubility :Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc.
form :Powder
color :White to yellow
solubility :Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc.
form :Powder
color :White to yellow
Safety Information
| Symbol(GHS): | ||||||||
|---|---|---|---|---|---|---|---|---|
| Signal word: | ||||||||
| Hazard statements: |
|
|||||||
| Precautionary statements: |
|
Description
Fmoc-Lys(Me)3-OH chloride is one of the common N-terminal protected reagents used in the peptide synthesis. Some of the reported examples are:Synthesis of peptide linkers for monoclonal antibody-auristatin F conjugates.
Preparation of multifunctional reagents with enhanced ionization properties for the analysis of protein modification in human cells and dynamic profiling of protein lipidation.
Sequential peptide ligation to synthesize histone H3 containing a trimethyl lysine residue, with modified tail region.
Related product price
- FMOC-LYS(ME)2-OH HCL
₹16390.01-120220 - FMOC-LYS(BOC)(ME)-OH
₹29810.01-365680 - FMOC-LYS(DNP)-OH
₹26810-288119.99






