Pentobarbital sodium
|
- ₹0
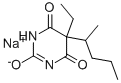
- Product name: Pentobarbital sodium
- CAS: 57-33-0
- MF: C11H17N2NaO3
- MW: 248.25
- EINECS:200-323-9
- MDL Number:MFCD00070198
- Synonyms:Pentobarbital sodium salt,5-Ethyl-5-(1-methylbutyl)-2,4,6-trioxohexahydropyrimidine, Nembutal;2,4,6(1H,3H,5H)-PyriMidinetrione,5-ethyl-5-(1-Methylbutyl)-, sodiuM salt (1:1);barpe;PENTOBARBITAL SODIUM;5-ethyl-5-(1-methylbutyl)-2,4,6(1h,3h,5h)-pyrimidinetrionemonosodiumsalt ;5-Ethyl-5-(1-Methylbutyl)-;5-ethyl-5-(1-methylbutyl)barbituricacidsodiumsalt ;5-ethyl-5-(1-methylbutyl)-barbituricacisodiumsalt
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :127 °C (decomp)
solubility :H2O: soluble
form :Solid
color :Off-White
Water Solubility :Soluble to 100 mM in water
CAS DataBase Reference :57-33-0(CAS DataBase Reference)
EPA Substance Registry System :Pentobarbital sodium (57-33-0)
solubility :H2O: soluble
form :Solid
color :Off-White
Water Solubility :Soluble to 100 mM in water
CAS DataBase Reference :57-33-0(CAS DataBase Reference)
EPA Substance Registry System :Pentobarbital sodium (57-33-0)
Safety Information
| Symbol(GHS): |

|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Danger | ||||||||||||||||
| Hazard statements: |
|
||||||||||||||||
| Precautionary statements: |
|
Description
Barbituric acid, the precursor of barbiturates, was first produced in 1864 by condensation of malonic acid and urea; it had no central nervous system (CNS) effects. In 1903, diethyl barbituric acid (barbital) was created as the first barbiturate with CNS inhibitory effects. Barbiturates were commonly used as sedative-hypnotics in the mid-twentieth century; meantime they were abused by some people as sold on the street. Use of barbiturates quickly dropped after introduction of benzodiazepines as the safer sedative-hypnotics. However some of the barbiturates are still used as anticonvulsants and some for euthanasia.More related product prices
144-02-5 Aluminum oxide Ethyl cyanoacetate Sodium alginate Ethylparaben Diantimony trioxide Ethyl propiolate Ethyl acetate Sodium acetate Sodium benzoate Diclofenac sodium Etanol Sodium hydroxide Trinexapac-ethyl Ethyl acrylate Sodium bicarbonate Sodium citrate Trisodium phosphateRelated product price
- Aluminum oxide
₹430-197365 - Ethyl cyanoacetate
₹790-10929 - ISOXADIFEN-ETHYL
₹9500-17698.88






