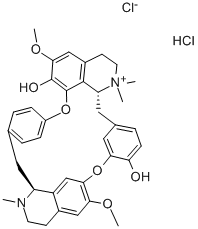
D-TUBOCURARINE CHLORIDE
|
- ₹3459.99
- Product name: D-TUBOCURARINE CHLORIDE
- CAS: 57-94-3
- MF: C37H41N2O6.ClH.Cl
- MW: 681.65
- EINECS:200-356-9
- MDL Number:MFCD00013471
- Synonyms:(+)-tubocurarinehydrochloride ;amerizol ;d-tubocurarinehydrochloride ;intocostrin ;intocostrinet ;tubadil ;tubarine ;tubocurarinehydrochloride
1 prices
Selected condition:
Brand
- Sigma-Aldrich(India)
Package
- 10MG
- ManufacturerSigma-Aldrich(India)
- Product number5.05145
- Product description(+)-Tubocurarine Chloride - CAS 57-94-3 - Calbiochem
- Packaging10MG
- Price₹3459.99
- Updated2022-06-14
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 5.05145 | (+)-Tubocurarine Chloride - CAS 57-94-3 - Calbiochem | 10MG | ₹3459.99 | 2022-06-14 | Buy |
Properties
Melting point :274~275℃
alpha :D20-25 +215° (c = 0.25-0.3 g/100 ml)
Density :1.2074 (rough estimate)
refractive index :1.7350 (estimate)
storage temp. :2-8°C
solubility :Soluble to 25 mM in water and to 10 mM in DMSO
form :Powder
pka :pK: 7.4(at 25℃)
color :white
Water Solubility :Soluble to 25 mM in water
alpha :D20-25 +215° (c = 0.25-0.3 g/100 ml)
Density :1.2074 (rough estimate)
refractive index :1.7350 (estimate)
storage temp. :2-8°C
solubility :Soluble to 25 mM in water and to 10 mM in DMSO
form :Powder
pka :pK: 7.4(at 25℃)
color :white
Water Solubility :Soluble to 25 mM in water
Safety Information
| Symbol(GHS): |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Danger | ||||||||||||||
| Hazard statements: |
|
||||||||||||||
| Precautionary statements: |
|
Description
The name curare is derived from the native Guyana Mukusi Indian word wurari. In 1596, Sir Walter Raleigh referred to curare in The Discovery of the Large, Rich, and Beautiful Empire of Guiana. In 1780, Abbe Felix Fontana identified the action of curare on voluntary muscles. In 1800, Alexander von Humboldt described the extraction of curare. In 1811, Sir Benjamin Collins Brodie determined that complete recovery from curare poisoning is possible provided artificial ventilation is maintained. In 1825, Charles Waterton brought curarep to Europe, and in 1835 Sir Robert Hermann Schomburgk classified and named the vine Strychnos toxifera. In 1850, George Harley demonstrated that curare could be used to treat tetanus and strychnine poisoning. By 1868, Claude Bernard and Alfred Vulpian had identified the site of action of curare as the motor end plate. From 1887, curare was marketed for medical use by Burroughs Welcome. In 1900, Jacob Pal recognized that physostigmine could be used to antagonize the effects of curare. In 1912, Arthur Lawen demonstrated the use of curare during surgery, but this potential was not realized as the finding was published in German. In 1914, Henry Hallett Dale described the action of acetylcholine. In 1935, Harold King isolated D-tubocurarine and described its structure, while in 1936 Dale revealed the role of acetylcholine in neuromuscular transmission and the mechanism of action for curare. In 1940, Abram Elting Bennett revealed that curare could be used to reduce trauma during metrazol-induced convulsive therapy for spastic disorders in children. In 1942, Harold Griffith and Enid Johnson used curare to augment general anesthesia when performing an appendectomy. Curare was used surgically until the development of safer synthetic neuromuscular blocking analogues such as Pancuronium (in 1964), Vecuronium (in 1979), Mivacurium (in 1993), and Rocuronium (in 1994).More related product prices
4-Methoxyphenethylamine 2328-12-3 19342-01-9 3-PHENOXYPHENOL 17279-31-1 Benzyltrimethylammonium chloride 645-33-0Related product price
- 41789-95-1
₹4817.13-20513.38 - 4-Methoxyphenethylamine
₹5401.68-29800 - 2328-12-3
₹1807.78-20773






