Favipiravir
|
- ₹0
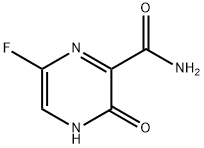
- Product name: Favipiravir
- CAS: 259793-96-9
- MF: C5H4FN3O2
- MW: 157.1
- EINECS:1533716-785-6
- MDL Number:MFCD12032148
- Synonyms:Pyrazinecarboxamide, 6-fluoro-3,4-dihydro-3-oxo- (9CI);Favipiravir;Unii-ew5gl2X7E0;6-fluoro-3,4-dihydro-3-oxo- (9CI);PYRAZINECARBOXAMIDE, 6-FLUORO-3,4-DIHYDRO-3-OXO-;2-PyrazinecarboxaMide, 6-fluoro-3,4-dihydro-3-oxo-;6-Fluoro-3-oxo-3,4-dihydropyrazine-2-carboxaMide;PyrazinecarboxaMide, 6-fl...
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :>151°C (dec.)
Density :1.78±0.1 g/cm3(Predicted)
storage temp. :under inert gas (nitrogen or Argon) at 2-8°C
solubility :Soluble in DMSO (30 mg/mL), water (12 mg/mL with warming)
form :solid
pka :8.77±0.60(Predicted)
color :Off-white
Stability :Stable for 1 year from date of purchase as supplied. Solutions in DMSO or water may be stored at -20° for up to 3 months.
Density :1.78±0.1 g/cm3(Predicted)
storage temp. :under inert gas (nitrogen or Argon) at 2-8°C
solubility :Soluble in DMSO (30 mg/mL), water (12 mg/mL with warming)
form :solid
pka :8.77±0.60(Predicted)
color :Off-white
Stability :Stable for 1 year from date of purchase as supplied. Solutions in DMSO or water may be stored at -20° for up to 3 months.
Safety Information
| Symbol(GHS): |

|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | ||||||||||||||
| Hazard statements: |
|
||||||||||||||
| Precautionary statements: |
|
Description
Favipiravir is a new broad-spectrum antiviral drug targeting RNA-dependent RNA polymerase (RdRp) developed by Japan's Toyama Chemical Pharmaceutical Company. It was approved for marketing in Japan in March 2014 for the treatment of new and recurrent influenza. During the outbreak of the new coronavirus, the results of the Phase I clinical study of the drug published in March 2020 showed that the drug may have the effect of speeding up virus clearance to alleviate the progress of new coronavirus pneumonia.More related product prices
6-Fluoro-1H-indole-4-carboxylic acid methyl ester 116293-90-4 Velpatasvir 57226-68-3 AZ20 METHOPRENE ACIDRelated product price
- 116293-90-4
₹27787.78 - METHOPRENE ACID
₹35235.38






