pilaralisib
|
- ₹0
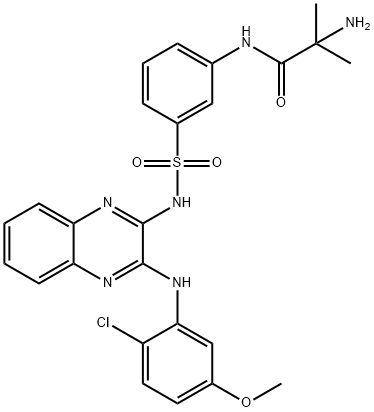
- Product name: pilaralisib
- CAS: 934526-89-3
- MF: C25H25ClN6O4S
- MW: 541.02
- EINECS:
- MDL Number:MFCD26142605
- Synonyms:PILARALISIB (XL147);SAR245408;XL 147;XL-147,Pilaralisib, SAR245408;XL147(pilaralisib);2-amino-N-[3-[[3-(2-chloro-5-methoxyanilino)quinoxalin-2-yl]sulfamoyl]phenyl]-2-methylpropanamide;2-Amino-N-[3-[N-[3-[(2-chloro-5-methoxyphenyl)amino]quinoxalin-2-yl]sulfamoyl]phenyl]-2-methylpropanamide;Pilaralisib (XL-147);pilaralisib;Propanamide, 2-amino-N-[3-[[[3-[(2-chloro-5-methoxyphenyl)amino]-2-quinoxalinyl]amino]sulfonyl]phenyl]-2-methyl-
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|
Properties
Melting point :216-220oC
Density :1.458±0.06 g/cm3(Predicted)
storage temp. :Refrigerator
solubility :DMSO (Slightly)
pka :6.33±0.30(Predicted)
form :Solid
color :Light Yellow to Yellow
Density :1.458±0.06 g/cm3(Predicted)
storage temp. :Refrigerator
solubility :DMSO (Slightly)
pka :6.33±0.30(Predicted)
form :Solid
color :Light Yellow to Yellow
Safety Information
| Symbol(GHS): |
 
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Danger | |||||||||||||||||||||
| Hazard statements: |
|
|||||||||||||||||||||
| Precautionary statements: |
|
Description
The phosphatidylinositol 3-kinase (PI3K) signaling pathway has central roles in cell growth, development, and survival. Pilaralisib is a methylbenzenesulfonamide derivative that reversibly inhibits class I PI3Ks (IC50s = 39, 36, 23, and 383 nM for p110α, -δ, -γ, and -β, respectively). It has been shown to abrogate Akt and S6 kinase phosphorylation, reduce cyclin D1 and retinoblastoma protein, and upregulate the CDK inhibitor p27, inhibiting cell proliferation. In HER2-overexpressing breast cancer cells, cotreatment with the HER2 inhibitors trastuzumab or lapatinib are reported to sensitize tumor cells to the growth inhibitory effect of pilaralisib. This compound has been evaluated in clinical trials in patients with advanced solid tumors.Related product price
- XL019
₹7230-29170 - 13244-33-2
₹3700-22100 - 3-(3,4-Difluorobenzoyl)-1,2,3,6-tetrahydro-1,1-dimethylazepino[4,5-b]indole-5-carboxylic acid 1-methylethyl ester
₹10825-43613.93






