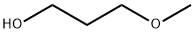1,3-Dimethoxypropane synthesis
- Product Name:1,3-Dimethoxypropane
- CAS Number:17081-21-9
- Molecular formula:C5H12O2
- Molecular Weight:104.15
Yield:-
Reaction Conditions:
with water;sulfuric acid at 220; for 10 h;Product distribution / selectivity;
Steps:
2.1
Comparative Example 2-1; In a stainless steel autoclave having an inner volume of 30 ml (manufactured by Taiatsu Techno Corporation, in Teflon pestle) including a stirrer, 0.10 g of sulfuric acid, 5.00 g of deionized water and 1.00 g of 3-methoxy-l-propanol were charged, and then an apparatus was assembled. After closing a vessel, an air in the autoclave was replaced with nitrogen by repeating an operation of pressurizing the autoclave to 1.0 MPa (gauge pressure) with nitrogen and depressurizing to 0.0 MPa (gauge pressure) five times. The contents were heated while stirring with a magnetic stirrer and then reacted at 220°C for 10 hours. After the completion of the reaction, the vessel was cooled to room temperature and depressurized. After opening a reactor, the supernatant was sampled and analyzed by GC. The results calculated by GC chromatogram are shown in Table 2-1 described hereinafter. GC revealed that peaks assigned to carbonyl compounds such as acrolein, 3-allyloxy-1-propionaldehyde and 3-hydroxy-1-propionaldehyde were not detected (these carbonyl compounds showed GC detection limit of 10 ppm or less in this example), but 1, 3-dimethoxypropane was produced.
References:
SHOWA DENKO K.K. WO2005/75392, 2005, A2 Location in patent:Page/Page column 64-65
115-10-6
92 suppliers
$75.00/25 g
110-71-4
472 suppliers
$13.00/25ml

17081-21-9
39 suppliers
inquiry

20637-49-4
14 suppliers
inquiry