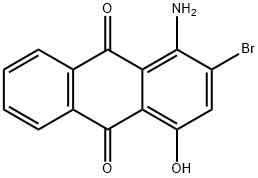
1-Amino-2-bromo-4-hydroxy-9,10-anthraquinone synthesis
- Product Name:1-Amino-2-bromo-4-hydroxy-9,10-anthraquinone
- CAS Number:116-82-5
- Molecular formula:C14H8BrNO3
- Molecular Weight:318.12
82-45-1
291 suppliers
$9.00/25g

116-82-5
119 suppliers
$56.00/250mg
Yield: 89.5%
Reaction Conditions:
with bromine;boric acid in water
Steps:
1 EXAMPLE 1
EXAMPLE 1 9.0 kg of bromine are added to a mixture of 10.0 kg of 1-aminoanthraquinone (97%) and 15 l of 98% strength sulphuric acid at 50° C. in a pressure vessel. The reaction vessel is then tightly closed and the contents of the vessel are vigorously stirred at 70° C. for 1 hour, at 80° C. for 1 hour and finally at 90° C. for 2 hours. The vessel is then cooled to 50° C. and the pressure is slowly released. Without intermediate isolation, 5.5 kg of boric acid and 27 l of oleum (20%) are added consecutively and cautiously to the bromination mixture, the temperature being maintained at 50°-60° C. with cooling, before it is heated at 75° C. (1 hour) and then at 90° C. and 100° C. for 1 hour each. It is finally heated to 115° C. and stirred at this temperature for 6 hours, the bromine which is produced distilling out. After cooling to 30°-40° C., excess bromine is removed in vacuo, and the reaction mixture is discharged onto 80 l of water. During this, the temperature rises to 90° C. The temperature is increased to 95° C. by blowing in steam. The mixture is allowed to stir at this temperature for 2 hours, and then the product is filtered off through a suction filter. After washing with hot water and drying, 12.4 kg--converted to 100% pure material--of 1-amino-2-bromo-4-hydroxyanthraquinone are obtained, which corresponds to a yield of 89.5%--the product contains less than 0.5% of 1-amino-4-hydroxyanthraquinone and about 1% each of 1-amino-2,4-dihydroxyanthraquinone and 1-amino-2,4-dibromoanthraquinone, together with traces of other bromo derivatives.
References:
Bayer Aktiengesellschaft US4741867, 1988, A
116-85-8
110 suppliers
$19.00/25mg

116-82-5
119 suppliers
$56.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

116-82-5
119 suppliers
$56.00/250mg
![3-bromo-5-hydroxy-6H-anthra[1,9-cd]isoxazol-6-one](/CAS/20180703/GIF/82827-33-6.gif)
82827-33-6
1 suppliers
inquiry

116-82-5
119 suppliers
$56.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

116-82-5
119 suppliers
$56.00/250mg