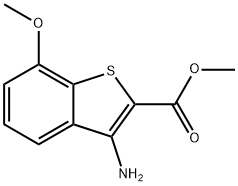
Methyl 3-amino-7-methoxybenzo[b]thiophene-2-carboxylate synthesis
- Product Name:Methyl 3-amino-7-methoxybenzo[b]thiophene-2-carboxylate
- CAS Number:198204-08-9
- Molecular formula:C11H11NO3S
- Molecular Weight:237.27

2365-48-2
339 suppliers
$14.00/25g
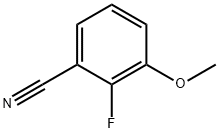
198203-94-0
81 suppliers
inquiry
![Methyl 3-amino-7-methoxybenzo[b]thiophene-2-carboxylate](/CAS/20180703/GIF/198204-08-9.gif)
198204-08-9
11 suppliers
inquiry
Yield:198204-08-9 94%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 1.5 h;Inert atmosphere;
Steps:
A.A.1.A.1.12.A.1.12.7 A.1.12.7. Methyl 3-amino-7-methoxybenzo[b]thiophene-2-carboxylate
To a mixture of 2-fluoro-3-methoxybenzonitrile (7.000 g, 45.90 mmol) and potassium carbonate (12.802 g, 91.70 mmol) in DMF (50 mL) is added dropwise methyl 2-mercaptoacetate (4.53 mL, 48.10 mmol). The RM is stirred at RT, under nitrogen, for 1.5h. Water is then added, and the resulting suspension is filtered. The separated solid is then washed with water and dried under high vacuum to give methyl 3-amino-7- methoxybenzo[b]thiophene-2-carboxylate as a beige solid (10.200 g, 94%). LC-MS B: tR = 0.90 min; [M+H]+ = 238.07.
References:
WO2018/210987,2018,A1 Location in patent:Page/Page column 87
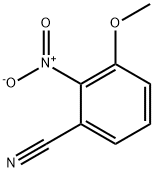
142596-50-7
68 suppliers
inquiry

2365-48-2
339 suppliers
$14.00/25g
![Methyl 3-amino-7-methoxybenzo[b]thiophene-2-carboxylate](/CAS/20180703/GIF/198204-08-9.gif)
198204-08-9
11 suppliers
inquiry
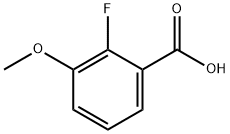
137654-20-7
151 suppliers
$8.00/1g
![Methyl 3-amino-7-methoxybenzo[b]thiophene-2-carboxylate](/CAS/20180703/GIF/198204-08-9.gif)
198204-08-9
11 suppliers
inquiry
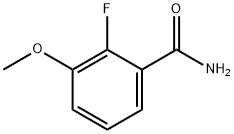
198204-64-7
9 suppliers
inquiry
![Methyl 3-amino-7-methoxybenzo[b]thiophene-2-carboxylate](/CAS/20180703/GIF/198204-08-9.gif)
198204-08-9
11 suppliers
inquiry