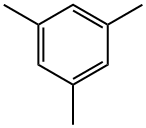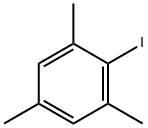
2,4,6-Trimethyliodobenzene synthesis
- Product Name:2,4,6-Trimethyliodobenzene
- CAS Number:4028-63-1
- Molecular formula:C9H11I
- Molecular Weight:246.09
A 10 mL glass flask equipped with a magnetic stir bar wascharged with a methyl ketone (1 mmol) and dissolved in MeCN (2 mL). To thermostat solution NH4NO3 (10-25 mol%), I2 (50 mol%) and H2SO4 (aqueous 96% solution, 10-20 mol%) were added and the flask was furtherequipped with a balloon filled with air (1 L) and magnetically stirred at 60 C.The consumption of starting material was monitored by TLC. After completion of the reaction, the mixture was cooled to room temperature, diluted withEtOAc (10 mL), and insoluble material identified as ammonium sulfate filtered off. The filtrate neutralized with NaHCO3 (aqueous 10% solution, 2 mL)and Na2S2O3 (aqueous 10% solution, 2 mL) and phase was separated. The waterphase was washed with EtOAc additionally two times (2 5 mL). The combined organic phase was dried over anhydrous Na2SO4 and the solvent distilled under reduced pressure. The crude product obtained was analyzed by 1H NMR. Finally, the crude product was purified using column chromatography (SiO2, n-hexane/CH2Cl2 elution) and preparative thin layer chromatography to afford purematerial.

Yield:4028-63-1 4% ,53779-84-3 96%
Reaction Conditions:
with [bis(acetoxy)iodo]benzene;iodine in ethyl acetate at 60; for 16 h;
References:
Togo, Hideo;Nogami, Genki;Yokoyama, Masataka [Synlett,1998,# 5,p. 534 - 536]

88-05-1
314 suppliers
$6.00/1g

4028-63-1
170 suppliers
$9.00/1g
139139-80-3
33 suppliers
$117.00/1G

105-67-9
295 suppliers
$14.00/1g

4028-63-1
170 suppliers
$9.00/1g
1268694-21-8
0 suppliers
inquiry
480-63-7
324 suppliers
$6.00/5g

4028-63-1
170 suppliers
$9.00/1g

1416277-01-4
1 suppliers
inquiry

4028-63-1
170 suppliers
$9.00/1g
2050-67-1
49 suppliers
$505.36/5MG