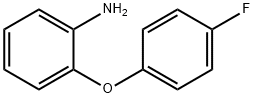
2-(4-FLUOROPHENOXY)ANILINE synthesis
- Product Name:2-(4-FLUOROPHENOXY)ANILINE
- CAS Number:3169-71-9
- Molecular formula:C12H10FNO
- Molecular Weight:203.21
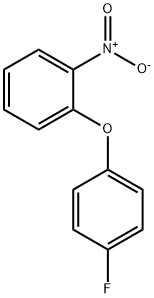
4475-59-6
6 suppliers
inquiry

3169-71-9
20 suppliers
$275.00/1g
Yield:3169-71-9 75.9%
Reaction Conditions:
with hydrogenchloride;tin(II) chloride dihdyrate in tetrahydrofuran;water at 20 - 40; for 2 h;
Steps:
18.2 <2> Synthesis of 1-(4-fluorophenoxy)-2-aniline
510 mL of concentrated hydrochloric acid and 510 g (2.26 mol) of tin chloride dihydrate were placed in a 2 L four-necked round bottom flask equipped with a stirrer, an Erlin condenser, a 1 L dropping funnel and a thermometer, and the mixture was stirred at room temperature. Next, a solution of 120.0 g (0.51 mol) of the nitro compound obtained in the above in 510 mL of THF was added dropwise with thorough stirring. Heat generation began almost simultaneously with the start of the dropwise addition, so when it exceeded 40 ° C., it was cooled in a water bath. After completion of the dropwise addition, the dropping funnel was washed with 50 mL of THF, added to the reaction solution, postreacted at 40 ° C. for 2 hours, and then cooled to room temperature.The obtained reaction solution was transferred to a 3 L four-necked round bottom flask equipped with a stirrer, a Liebig condenser, a 500 mL dropping funnel and a thermometer, cooled to 5 ° C. with an ice water bath, and 40% aqueous sodium hydroxide solution 285 mL was added dropwise while paying attention to heat generation to make it alkaline. The precipitated inorganic salt was removed by suction filtration, and the residue was washed with 1 L of ethyl acetate. Next, the filtrate was transferred to a 3 L separatory funnel, the aqueous layer was separated and extracted with 500 mL of ethyl acetate. Next, the ethyl acetate layers were combined into one, washed with 500 mL of water and dried with potassium carbonate, then the potassium carbonate was removed by suction filtration and the solvent was distilled off under reduced pressure. Subsequently, the obtained crude oil was purified by silica gel column chromatography using toluene as a developing solvent to obtain 78.6 g (yield 75.9%) of the objective compound.
References:
JP2018/90561,2018,A Location in patent:Paragraph 0083

371-41-5
512 suppliers
$8.00/25g

3169-71-9
20 suppliers
$275.00/1g

1493-27-2
506 suppliers
$5.00/10g

3169-71-9
20 suppliers
$275.00/1g
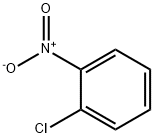
88-73-3
8 suppliers
$16.00/25g

3169-71-9
20 suppliers
$275.00/1g