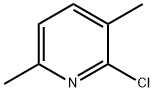
2-Chloro-3,6-dimethylpyridine synthesis
- Product Name:2-Chloro-3,6-dimethylpyridine
- CAS Number:72093-14-2
- Molecular formula:C7H8ClN
- Molecular Weight:141.6
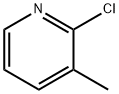
18368-76-8
321 suppliers
$8.00/1g

74-88-4
340 suppliers
$15.00/10g

72093-14-2
33 suppliers
$72.00/100mg
Yield: 60%
Reaction Conditions:
Stage #1: with n-butyllithium;2-(N,N-dimethylamino)ethanol in hexane at -5; for 0.5 h;
Stage #2:2 chloro-3-methylpyridine in hexane at -75 - -70; for 1.5 h;
Stage #3:methyl iodide in tetrahydrofuran;hexane at -70 - 0;
Steps:
A -50C solution of 2-dimethylaminoethanol (2.15g, 24.1 mmol) in hexane (15.0 mL) was slowly treated with n-butyllithium (1.4M, 35.0 mL, 49.0 mmol) and stirred for 30 minutes. The mixture was cooled to -750C and a solution of 2-chloro-3-methylpyridine (1.02g, 8.0 mmol) in hexane (15.0 mL) was slowly added maintaining the temperature at < -7O0C. After 1.5 hours, a solution of iodomethane (2.0 mL, 32.1 mmol) in THF (60.0 mL) was added slowly, maintaining the temperature at < -7O0C. The cold bath was removed and the mixture warmed to O0C. Water (60.0 mL) was carefully added, the layers separated, and the aqueous portion extracted with diethyl ether. The combined organic portions were washed (water, brine), dried (MgSO4), and concentrated to a crude oil that was EPO
References:
Location in patent:Page/Page column 147; 149-150

18368-76-8
321 suppliers
$8.00/1g
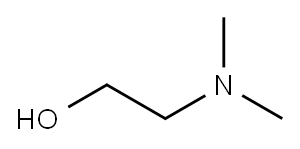
108-01-0
7 suppliers
$10.00/20mg

74-88-4
340 suppliers
$15.00/10g

72093-14-2
33 suppliers
$72.00/100mg
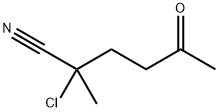
125014-90-6
0 suppliers
inquiry

72093-14-2
33 suppliers
$72.00/100mg