3-AMINOHEPTANE synthesis
- Product Name:3-AMINOHEPTANE
- CAS Number:28292-42-4
- Molecular formula:C7H17N
- Molecular Weight:115.22
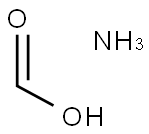
4164-92-5
7 suppliers
inquiry

28292-42-4
20 suppliers
inquiry
Yield:28292-42-4 66%
Reaction Conditions:
with trichloroisocyanuric acid;sodium hydroxide in water;acetonitrile at 0; for 1.5 h;Reflux;Hofmann Rearrangement;Reagent/catalyst;
Steps:
General procedures for the Hofmann reaction mediated by trihaloisocyanuricacids.
General procedure: (a) Carboxamides: A solution of the carboxamide (20 mmol) and NaOH(4.4 g, 110 mmol) in H2O (15 mL) and MeCN (25 mL) was poured into a coldsuspension of the trihaloisocyanuric acid (6.7 mmol) in water (10 mL), stirredfor 1 h at 0 oC and then heated under reflux for 30 min. After cooling, the solid(cyanuric acid) was filtered off, and the filtrate was extracted withdichloromethane (3 x 20 mL). The combined layers were dried (Na2SO4)and the solvent evaporated to give the amine.
References:
Bastos, Gustavo A.;de Mattos, Marcio C.S. [Tetrahedron Letters,2021,vol. 83,art. no. 153422]
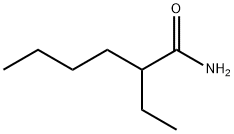
106-35-4
136 suppliers
$32.00/25mL

28292-42-4
20 suppliers
inquiry
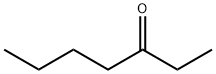
2108-81-8
1 suppliers
inquiry

28292-42-4
20 suppliers
inquiry
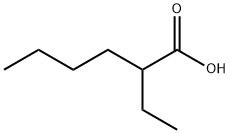
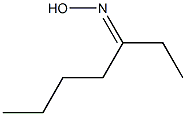
149-57-5
523 suppliers
$5.00/25g

28292-42-4
20 suppliers
inquiry