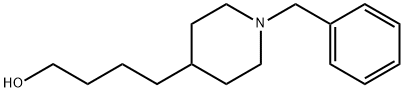
4-(1-Benzylpiperidin-4-yl)butan-1-ol synthesis
- Product Name:4-(1-Benzylpiperidin-4-yl)butan-1-ol
- CAS Number:318508-02-0
- Molecular formula:C16H25NO
- Molecular Weight:247.38

57614-92-3
64 suppliers
$79.00/100mg

100-39-0
425 suppliers
$10.00/10g

318508-02-0
15 suppliers
inquiry
Yield:318508-02-0 65%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in acetonitrile at 105; for 1 h;
Steps:
16 4.1.16
1-Benzyl-4-(4-hydroxybutyl)piperidine (26)
In two 20-mL pressure vials (20 bar) amine 25 (2 * 4.0 g, 2 * 25.45 mmol) was dissolved in acetonitrile (2 * 10 mL) under warming.
Diisopropylethylamine (2 * 3.62 g, 2 * 28.0 mmol) and benzyl bromide (2 * 4.78 g, 2 * 28.0 mmol) were added under stirring and the mixture was heated in a bath at 105 °C for 60 min.
The solvent was removed under reduced pressure and the residue taken up in CH2Cl2 (250 mL) and 5% aq NaOH (150 mL).
The mixture was vigorously shaken, the phases were separated, and the aqueous phase treated with CH2Cl2 (100 mL).
The CH2Cl2 extracts were combined, washed with water (50 mL) and dried over Na2SO4.
The solvent was removed under reduced pressure and the product was purified by column chromatography (eluent: CH2Cl2 to CH2Cl2/MeOH 10:1).
Amino alcohol 26 was obtained as an orange oil (8.2 g, 65%). Rf = 0.5 (CH2Cl2/MeOH 5:1). IR (neat) 3330 br, 2925, 1495, 1455 cm-1. 1H NMR (300 MHz, CD3OD) δ (ppm) 1.17-1.33 (m, 5H), 1.34-1.46 (m, 2H), 1.48-1.59 (m, 2H), 1.72 (br d, 2H, J 9.6 Hz), 2.03 (br t, 2H, J 11.3 Hz), 2.92 (br d, 2H, J 11.8 Hz), 3.54 (s, 2H), 3.56 (t, 2H, J 6.5 Hz), 7.26-7.37 (m, 5H).
13C NMR (75 MHz, CD3OD) δ (ppm) 24.9, 33.8, 34.7, 37.6, 38.3, 55.7, 63.8, 65.3, 129.3, 130.1, 131.8, 139.0. MS (ESI, MeOH) m/z (%) 270 (7) [M+Na]+, 248 (100) [M+H]+. C16H25NO (247.4).
References:
Keller, Max;Tr?nkle, Christian;She, Xueke;Pegoli, Andrea;Bernhardt, Günther;Buschauer, Armin;Read, Roger W. [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 14,p. 3970 - 3990]
316362-85-3
0 suppliers
inquiry

318508-02-0
15 suppliers
inquiry
3612-20-2
493 suppliers
$6.00/10g

318508-02-0
15 suppliers
inquiry
![2-Butenoic acid, 4-[1-(phenylmethyl)-4-piperidinylidene]-, ethyl ester, (2E)-](/CAS/20210305/GIF/316362-81-9.gif)
316362-81-9
0 suppliers
inquiry

318508-02-0
15 suppliers
inquiry

111-29-5
356 suppliers
$5.00/25g

318508-02-0
15 suppliers
inquiry