
4-FLUORO-2,6-DIMETHYLPHENOL synthesis
- Product Name:4-FLUORO-2,6-DIMETHYLPHENOL
- CAS Number:2338-56-9
- Molecular formula:C8H9FO
- Molecular Weight:140.15
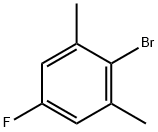
14659-58-6
127 suppliers
$8.00/1g

2338-56-9
48 suppliers
inquiry
Yield: 90%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);potassium hydroxide;tert-butyl XPhos in 1,4-dioxane;water at 100;Inert atmosphere;
Steps:
1.1g Example 1g
4-fluoro-2, 6-dimethylphenol
A 1L three-necked round-bottomed flask equipped with a magnetic stir bar was charged with di-tert-butyl (2', 4', 6'-triisopropyl- [1, 1'-biphenyl] -2-yl) phosphine (8.37 g, 19.70 mmol) , tris (dibenzylideneacetone) dipalladium (4.51 g, 4.92 mmol) and potassium hydroxide (41.4 g, 739 mmol) . The flask was evacuated and backfilled with nitrogen. Separately, dioxane (150 mL) , 2-bromo-5-fluoro-1, 3-dimethylbenzene (50 g, 246 mmol) and water (150 mL) were flow purged with nitrogen for about 30 minutes and were transferred to the reaction flask via a cannula. The reaction vessel was heated to about 100 C and stirred overnight. The reaction mixture was cooled to ambient temperature. The reaction mixture was acidified to pH 2 by adding 6N HCl and the product was extracted with dichloromethane (3 x 250 mL) . The combined organic layers were stirred with mercaptopropyl silica gel for about 30 minutes, dried over anhydrous magnesium sulfate, filtered, and concentrated to afford the title compound as a white solid. (31.2 g, 223 mmol, 90% yield)
References:
ABBVIE INC.;ABBVIE PHARMACEUTICAL TRADING (SHANGHAI) CO., LTD.;COWART, Marlon;FIDANZE, Steven;HASVOLD, Lisa;LIU, Dachun;MCDANIEL, Keith;PRATT, John;SHEPPARD, George;WANG, Le WO2018/68283, 2018, A1 Location in patent:Paragraph 00258
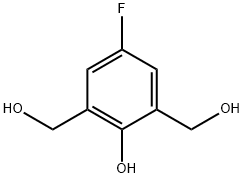
71643-58-8
15 suppliers
inquiry

2338-56-9
48 suppliers
inquiry
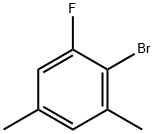
289038-20-6
16 suppliers
inquiry

2338-56-9
48 suppliers
inquiry

576-26-1
332 suppliers
$10.00/25g

2338-56-9
48 suppliers
inquiry

3095-38-3
79 suppliers
$50.00/1 g

2338-56-9
48 suppliers
inquiry